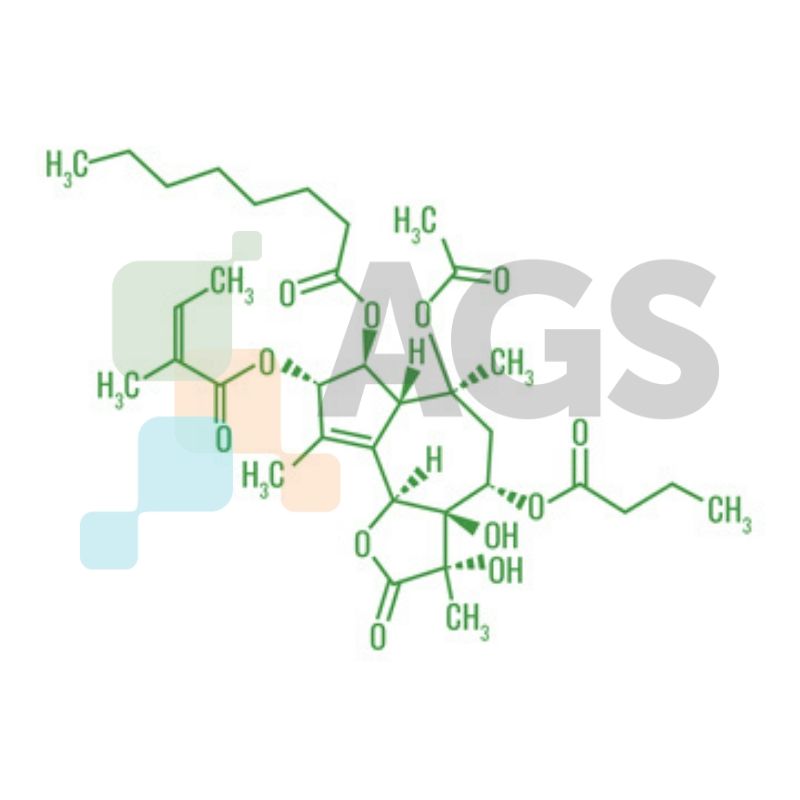
Thapsigargin is a sesquiterpene lactone that is known to inhibit sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA). Thapsigargin is derived from the plant Thapsia garganica that produces tumor promoters. This effect is a result of emptying the intracellular calcium stores, which leads to a chain of events that causes apoptosis.
Mechanism of Action
Thapsigargin is an inhibitor of sarco endoplasmic reticulum Ca2 ATPase (SERCA). It pumps calcium ions from the cytoplasm into the lumen of the endoplasmic reticulum (ER) and thapsigargin. This process will causes an increase in the cytoplasmic calcium levels while also depleting ER stores. More calcium will enter into the cells if there is an increase in cytoplasmic calcium. Thapsigargin also interferes with mechanisms of autophagy. It does this by inhibiting fusion of autophagosomes to lysosomes. The increased calcium levels, and dysregulated autophagy leads to ER stress,. The final result will lead to apoptosis and cell death.
Are thapsigargin and mipsagargin related?
Mipsagargin is an analog prodrug used as an anti-cancer therapeutic agent and diagnostic tool. The main target of mipsagargin is prostate specific membrane antigen (PSMA). The peptide ligand is attached to the thapsigargin analog molecule which is cleaved by PSMA. This process activates thapsigargin, triggering apoptosis of cancer cells. GenSpera company, is currently running trials on mipsagargin.Applications
- Used as tumor promoter and induces an increase in intracellular stores of Ca++ without hydrolysis.
- Used in cancer cancer treatment due to its ability to induce apoptosis.
- Used as a weak promoter of skin tumors in mice.
- Inhibition of SERCA pump
Future Research
Future research is being conducted on the Unfolded Protein Response (UPR) and Endoplasmic reticulum (ER) . Thapsigargin is used in this research because its an ER stress inducer. The ER is where folding of membrane and secreted proteins in the cell takes place. If this process is disrupted, then the ER stress inducer (Thapsigargin) will set off signaling pathways of UPR. This promotes cellular repair and maintains a productive protein folding environment.
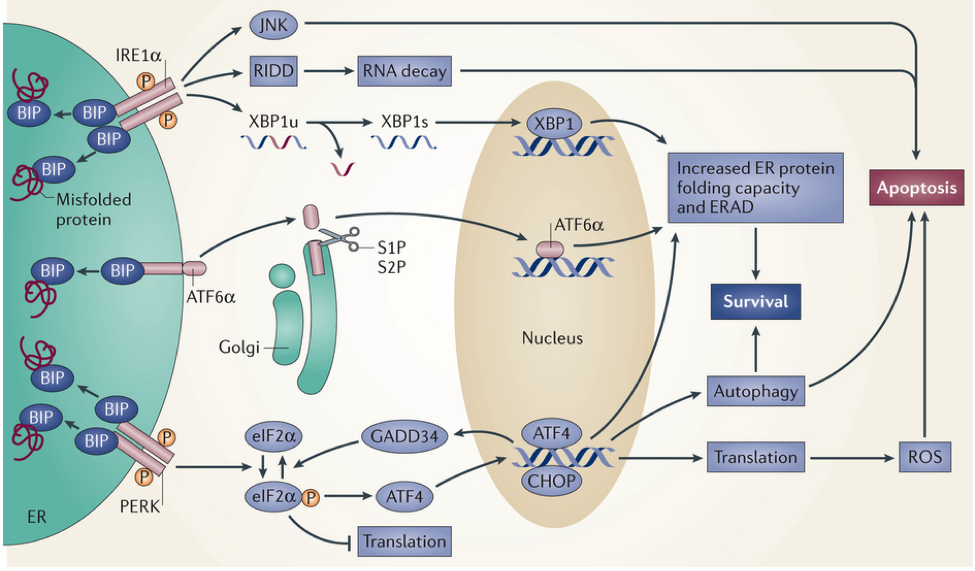
Upon endoplasmic reticulum (ER) stress, unfolded and misfolded proteins bind and sequester immunoglobulin heavy-chain binding protein (BIP), thereby activating the UPR. The UPR comprises three parallel signalling branches. The outcome of UPR activation increases protein folding, transport and ER-associated protein degradation (ERAD), while attenuating protein synthesis. If protein misfolding is not resolved, cells enter apoptosis.
Chemical Specifications
| Chemical Formula: | C34H50O12 |
| CAS Number: | 67526-95-8 |
| Molecular Weight: | 650.8 |
| Appearance: | White to off-white lyophilized powder |
| Purity: | ≥95% |
| Solubility: | Acetonitrile |
Recommended Concentrations for Usage
For a 1.25 mM stock, reconstitute 1 mg in 1.23 mL DMSO. Working concentrations and length of treatments vary depending on the desired effect, but it is typically used at 2-2000 nM for 0.5-24 hours.Thapsigargin Storage & Stability
Keep container tightly closed in a well-ventilated area. When handling use only in areas provided with appropriate exhaust ventilation. Keep away from heat and source of ignition. Empty containers pose a fire risk, evaporate residue under a fume hood. Ground all equipment containing material. Do not breathe dust. It is stable for approximately 2 years if stored at -20 °C. The above information is pertaining to AG Scientific. Other vendors may recommend different storage temperatures.Additional Reading
- Thapsigargin Applications: Glioblastoma Multiforme Studies
- In Vitro Mutagenesis for Genetic Research
- A Promising Cancer Therapy: Thapsigargin's Anti-Cancer Roles