AG Scientific is a leading supplier of EDAC HCl. Here we review some of the most frequently asked questions about this useful reducing agent.

What is EDAC HCl?
EDAC Hydrochloride is a versatile modern reducing agent. It is an easily handled solid with high solubility in water and organic solvents such as dichloromethane, tetrahydrofuran and dimethylformamide.
Are there alternative names?
-
EDCl
-
EDC hydrochloride
-
EDC HCl
-
Water Soluble Carbodiimide
-
WSC
-
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
-
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
What is the chemical formula?
C8H18ClN3
What is the molecular weight?
191.7
What does it look like?
AG Scientific's EDAC HCl is supplied as a white powder.
Is this product soluble?
EDCl is soluble in water and organic solvents including dichloromethane, tetrahydrofuran and dimethylformamide.
What is the mechanism of action?
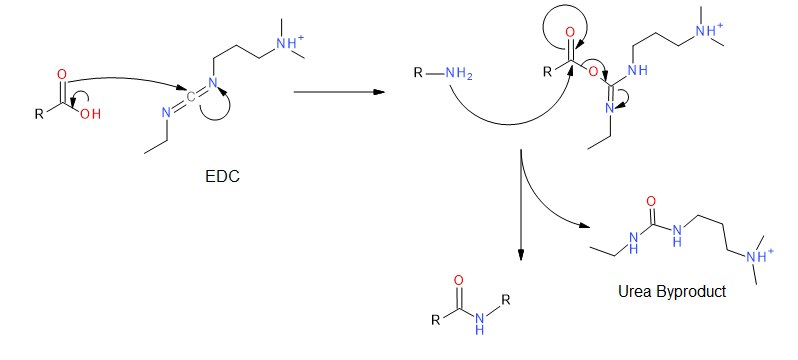
EDC couples primary amines to carboxylic acids by creating an activated ester leaving group. First, the carbonyl of the acid attacks the carbodiimide of EDC, and there is a subsequent proton transfer. The primary amine then attacks the carbonyl carbon of the acid which forms a tetrahedral intermediate before collapsing and discharging the urea byproduct. The desired amide is obtained.
What are applications of this reagent?
EDAC HCl is used for modifying NMDA receptors and is also used as a condensing agent in the synthesis of peptides. It is also being used increasingly in the production of new pharmaceuticals due to its simple purification process. EDC is often preferred to dicyclohexycarbodiimide (DCC) in peptide synthesis because of its ease of handling and the enhanced solubility of EDC and the urea by-product formed during the coupling reaction. The urea by-product is readily soluble in water and can easily be removed by extraction whereas the DCC by-product, dicyclohexylurea, is usually removed by multiple filtrations. EDAC hydrochloride is a useful reducing agent. Reducing agents have important industrial applications including:
-
Water purification
-
Textile manufacturing
-
Energy storage

