Oligomycin is an antibiotic that inhibits ATP synthase by blocking its proton channel (F0 subunit), which is necessary for oxidative phosphorylation of ADP to ATP (energy production). The inhibition of ATP synthesis would also stop electron transport chain. Because the high proton concentration build up is not dissipated, the free energy released by biological oxidation of substrates is not enough to pump any more protons against the steep gradient.
What is the orgin of Oligomycin?
Oligomycin was first identified in 1953 from a species called Streptomyces diastatochromogenes, but it has since been found in lots of other streptomycetes. It is synthesized in 1986 in Manchester, England. The researchers used simple carbon compounds (acetate, butyrate and propionate) labelled with carbon-13, fed these to the cells then extracted the oligomycin and looked at where the carbon-13 atoms were. In this way they could see that oligomycin is assembled piece by piece from smaller simple molecules containing carbon.
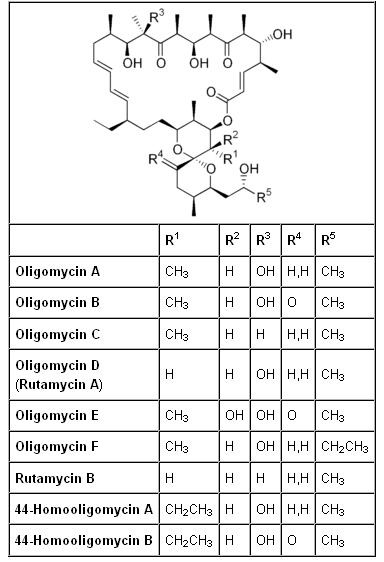
What is the bio-structure of Oligomycin?
What are the biochemical properties?
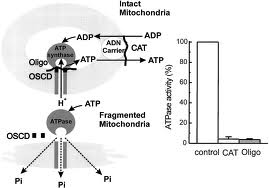
Various ion (proton)-translocating ATPases are present in prokaryotic and eukaryotic organisms. These ATPase are classified into three groups: F-type, V-type, and P-type (see ATPase). Mitochondrial H+-ATPase belongs to the F-type group and is called F-ATPase or ATP synthase in some cases. Other F-type H+-ATPases, present in thylakoid membranes of chloroplasts and inner membranes of bacteria, other than some photosynthetic bacteria, are oligomycin-insensitive. Na+, K+-ATPase belongs to the P-type group. In some cases, this ATPase is called the sodium pump. Other P-type ATPases, including Ca2+-ATPase and H+, K+-ATPase, localized in the sarcoplasmic reticulum and gastric cell membranes, respectively, are oligomycin-insensitive. V-type H+-ATPase, which is localized in membranes of subcellular particles in eukaryotic organisms, such as vacuoles and lysosomes, is also oligomycin-insensitive.
How does Oligomycin inhibit Mitochondrial H+-ATPase?
Mitochondrial H+-ATPase functions as an ATP synthase coupled to the respiratory electron flow in intact mitochondria. The yeast mitochondrial ATPase is less sensitive to oligomycin than the animal mitochondrial ATPase. The inhibitory potency of oligomycin is often expressed as an amount of oligomycin per milligram of ATPase: 95% inhibition is achieved by 0.4 g oligomycin/mg of bovine heart mitochondrial ATPase; 85% inhibition is achieved by 4 g oligomycin/mg of Neurospora crassa ATPase; and 90% inhibition is achieved by 10 g oligomycin/mg Saccharomyces cerevisiae ATPase.
F-type H+-ATPase is composed of two components: the F1 and F0 portions. The F1 portion is an oligomer composed of five different kinds of hydrophilic subunits named a, b, g, d, and e, and it includes the catalytic center for the synthesis or hydrolysis of ATP. The F0 portion is an integral membrane protein composed of three (bacteria) or more (eukaryotic organisms) different kinds of hydrophobic subunits, and it functions as a proton channel. The ATPase activity of the isolated F1 region is not inhibited by oligomycin.
The component that controls the oligomycin sensitivity of H+-ATPase has been purified from bovine heart mitochondrial ATPase and named oligomycin sensitivity-conferring protein(OSCP). However, OSCP does not contain a binding site for oligomycin; instead, it is one of the components required for correct binding between the F1 and F0 portions. The amino acid sequence of OSCP has homology with that of the d subunit in the F1 portion of bacterial H+-ATPase, which is insensitive to oligomycin.
Oligomycin-resistant mutants have been isolated from yeast and Chinese hamster ovary cell lines and analyzed genetically. The majority of the mutations were mapped at loci oli 1 to oli 4. The oli 2 and oli 4 loci were found in the gene for subunit 6 of F0, whereas the oli 1 and oli 3 were found in the gene for subunit 9 of F0. Some of the amino acids substituted in the mutants have been identified. In addition, the binding of dicyclohexylcarbodiimide (DCCD), another inhibitor of H+-ATPase, to subunit 9 is reduced by oligomycin.
In Escherichia coli H+-ATPase, which is insensitive to oligomycin, the region around oli 4 in the gene for subunit a, which corresponds to subunit 6, is deleted? suggesting that the binding site for oligomycin is absent in the E. coli protein. These results suggest that subunits 6 and 9 of the F0 portion determine the oligomycin sensitivity of F-type H+-ATPase. OSCP apparently affects the oligomycin sensitivity indirectly.
What is the solubility?
The oligomycins are stable compounds. Their inhibitory potency is little changed on storage at pH 3 to 10 at 37°C for 54 h. They are almost insoluble in water but are soluble in water-miscible solvents such as ethanol, methanol, dimethyl sulfoxide, dimethylformamide acetone, glacial acetic acid, and in ether. Oligomycins are usually dissolved in ethanol and stored in a refrigerator. Their maximum solubility in ethanol seems to be not more than 10 mM (about 8 mg/ml). Their solubility in water including 1% ethanol seems, from examination using [ H]-labeled oligomycin made in our laboratory and not commercially available, to be up to 10 mM (about 8 mg/mL).
What are the toxicities?
The LD50s of oligomycins A, B, and C in mice injected intraperitoneally are 1.5, 2.9, and 8.3 mg/kg of body weight, respectively. The corresponding inhibitory concentrations (IC50s) toward HeLa cells are 0.008, 0.015, and 0.106 mg/ml, respectively.


