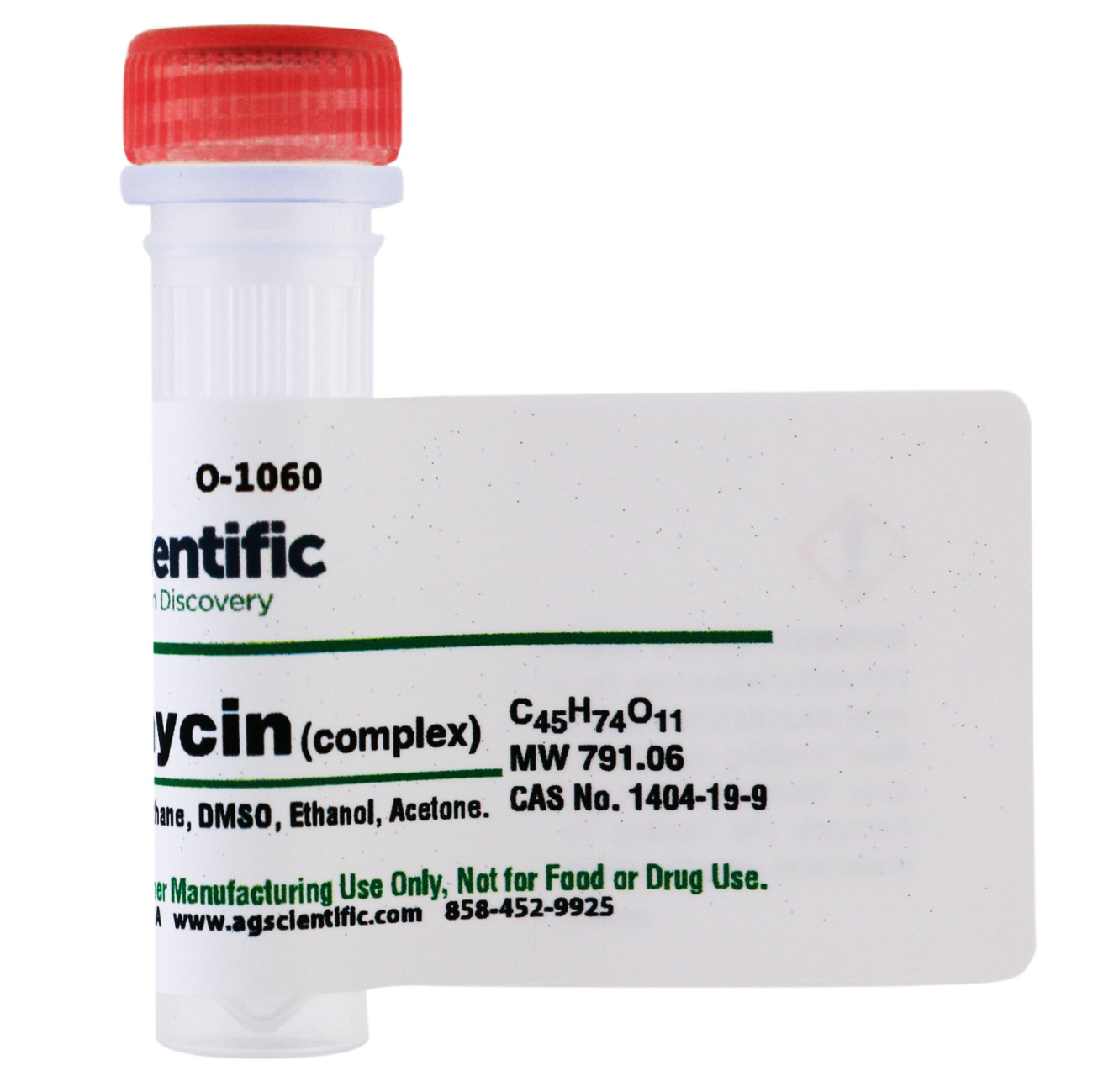
1404-19-9 (for Oligomycin A)
791.06 (for Oligomycin A)
C45H74O11 (for Oligomycin A)
Dichloromethane, DMSO, Ethanol, Acetone
-20°C
Oligomycin complex of Oligomycin A, B and C. This antibiotic inhibits membrane bound mitochondrial ATPase. It is isolated from Streptomyces diastatochromogenes. Inhibits mitochondrial F1F0 ATP synthase.
Oligomycin is a useful tool for decreasing cellular ATP levels. Induces autophagy. Stimulates lysosome acidification. Protects against ischemic kidney in male rats.
Additional Reading: Pathology Research Using Mitophagy Modulators
0.1 lbs
Research or further manufacturing use only, not for food or drug use.