Leupeptin is a protease inhibitor that is produced naturally by some species of the soil bacteria Streptomyces and thus inhibits the degradation of proteins. Unlike many synthetic protease inhibitors, it has very low toxicity to humans. This compound has been shown in research on animal models to protect hair cells in the ear from being killed by loud noise as well as certain antibiotics, and in turn prevents hearing loss.
What is Leupeptin?
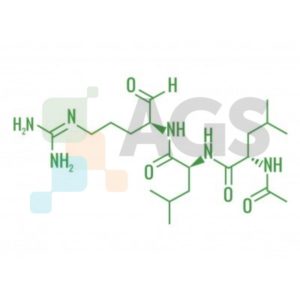 Leupeptin
Leupeptin is widely used during the first part of protein purifications to keep proteases in the tissue from degrading the protein of interest. This natural product is a small peptide, a molecule composed of three linked amino acids that have some unusual attributes. The amino group of the compound has an acetyl CH
3CH
2 group on it.
Additionally, the carboxy group is chemically different than most peptides and proteins, because it has an aldehyde group instead of a carbonyl moiety. Proteases fall into a number of classes. The first major distinction is whether they cleave in the middle of the molecule, like endopeptidases, or whether they nibble along at the ends of the molecules, like exopeptidases.
Leupeptin inhibits proteases with endopeptidase activity. There are also further classifications based on the structure of the active site, such as cysteine and serine proteases. This protease inhibitor prevents the activity of some of these types of enzymes, such as trypsin and
proteinase K, while also being ineffective with others such as the chymotrypsins.
1. Leupeptin as an anti-malarial agent
Leupeptin is a reversible inhibitor of serine and cysteine proteases. Leupeptin often used for inhibition of plasmin, trypsin, papain, kallikrein, cathepsin B, as well as, a novel antagonist of calpain. Leupeptin is also a lysosomal inhibitor. Administration of the protease inhibitor leupeptin concomitantly with mechanical ventilation completely prevented ventilation-induced diaphragmatic contractile dysfunction and atrophy. Cysteine protease inhibitors i.e. leupeptin kill malaria parasites and are being pursued to be development as antimalarial agents.
2. Leupeptin involvement in Parkinson's disease, Lewy bodies, and MSA
Abnormal accumulation of alpha-synuclein is regarded as a key pathological step in a wide range of neurodegenerative processes, not only in Parkinson's disease (PD) and dementia with Lewy bodies (DLB) but also in multiple-system atrophy (MSA). Nevertheless, the mechanism of alpha-synuclein accumulation remains unclear. Leupeptin, a protease inhibitor, has been known to cause various neuropathological changes in vivo resembling those of aging or neurodegenerative processes in the human brain, including the accumulation of neuronal processes and neuronal cytoskeletal abnormalities leading to neurofibrillary tangle (NFT)-like formations The present finding suggests that the local accumulation of alpha-synuclein might be induced by the impaired metabolism of alpha-synuclein, which are likely related to lysosomal or ubiquitin-independent proteasomal systems.
3. Leupeptin involvement in delaying hearing loss
This leupeptin study may serve as a basis for future clinical trials of leupeptin administration for the prevention or treatment of noise-induced hearing loss and the management of tinnitus. The goal of this study is to begin to define the effects of one of these agents--leupeptin, a calpain antagonist--on the normal inner ear of an animal model. In this investigation, we demonstrate the effects of sustained-release delivery of leupeptin (2.5 ug/mL) on the hearing of chinchillas. The medicine produced no hearing loss at the early time points but did produce some hearing loss at later time points.
4. Leupeptin effect on gingivitis disease
In this study, we developed a procedure to produce gingivitis in rats by inoculation of Porphyromonas gingivalis and studied the contribution of the bacterial cysteine proteinases, leupeptin. These results suggest that P. gingivalis-induced gingivitis is attributable to Rgp and Kgp and that leupeptin is more effective in the late phase than the early stage of gingivitis.
5. Leupeptin effect on neuronal development & regeneration
Fibroblast growth factors (FGFs) act as trophic factors during development and regeneration of the nervous system. We previously reported that the lysosomal inhibitor leupeptin prevents degradation of FGFR1 and promotes FGF-2-induced elongative axon growth of DRG neurons overexpressing FGFR1. Therefore, we analyzed the effects of leupeptin on the intracellular sorting of FGFR1 in PC12 pheochromocytoma cells and dorsal root ganglia (DRG) neurons. Leupeptin increased colocalization of FGFR1 with lysosomes. Together, our results strongly imply that increased recycling of FGFR1 promotes axon elongation, but not axonal branching, of adult DRG neurons in vitro.
6. Leupeptin connection to cardioplegia/reperfusion & nitric oxide synthase
The objective of this study was to investigate how long-term cardioplegia/reperfusion affects cardiac nitric oxide synthase 3 (NOS3). Only the treatment with leupeptin preserved NOS3, indicating that lysosomal proteases rather than cytoplasmic calpains were mainly responsible for the cleavage of this enzyme. The observed decrease in GSH/GSSG ratio and activation of JNK in the reperfused heart suggested that proteolysis could be triggered by reactive oxygen species.
7. Leupeptin effect on cellular stress & irradiation treatment
Proteases have received attention as important cellular components responsible for stress response in human cells. However, little is known about the role of proteases in the early steps of cell response after X-ray irradiation. Treatment of RSa cells with leupeptin prior to X-ray irradiation resulted in lowered colony survival and an increased ratio of G(2)/M-arrested cells and apoptotic cells. These results suggest that leupeptin-sensitive proteases are involved in the resistance of human RSa cells to X-ray cell-killing.
8. A leupeptin based assay for macroautophagy in mice.
Macroautophagy is a highly conserved catabolic process that is crucial for organ homeostasis in mammals. However, methods to directly measure macro autophagic activity (or flux) in vivo are limited. In this study, we developed a quantitative macro autophagic flux assay based on measuring LC3b protein turnover in vivo after administering the protease inhibitor leupeptin. Using this assay we then characterized basal macro autophagic flux in different mouse organs. We found that the rate of LC3b accumulation after leupeptin treatment was greatest in the liver and lowest in the spleen. These results illustrate the usefulness of our leupeptin-based assay for studying the dynamics of macroautophagy in mice.
9. Leupeptin role in Cardiac pathogenesis
In order to test the hypothesis that cardiac protein degradation contributes to the pathogenesis of myocardial stunning, the effect of protease inhibitor leupeptin on the postischemic hemodynamics and metabolic functioning was measured in isolated rat hearts. Leupeptin protects the heart from myocardial stunning, which is consistent with the idea that proteases contribute to the pathogenesis of this phenomenon.
10. Leupeptin relationship in muscle function & improves motoneuron survival
The effect of treatment with leupeptin, a calpain inhibitor, on motoneuron survival and muscle function, was examined in vitro and in vivo models of motoneuron degeneration. This improvement in motoneuron survival was reflected in a significant improvement in muscle function in the leupeptin-treated group. For example in the soleus muscle of treated rats 20.8 (+/-1.40 S.E.M., n=5) motor units survived compared with only 14.6 (+/-1.21 S.E.M., n=5) in untreated animals. Thus, treatment with leupeptin, a calpain inhibitor, rescues motoneurons from cell death and improves muscle function following nerve injury.
11. Leupeptin connection to neuro opioid receptors
It was reported that the ubiquitin-proteasome pathway is involved in agonist-induced down regulation of mu and delta opioid receptors [J. Biol. Chem. 276 (2001) 12345]. While evaluating the effects of various protease inhibitors on agonist-induced opioid receptor down regulation, we observed that while the peptide aldehyde, leupeptin (acetyl-L-Leucyl-L-Leucyl-L-Arginal), did not affect agonist-induced down regulation, leupeptin at submillimolar concentrations directly inhibited radioligand binding to opioid receptors. We propose that leupeptin inhibits ligand binding by reacting reversibly with essential sulfhydryl groups that are necessary for high-affinity ligand/receptor interactions.
Why is Leupeptin Important?
The breadth of protease inhibition by leupeptin makes it a very useful compound in biochemical research and large-scale protein production. Generally, when one starts to isolate a protein from a biological source, the disruption of the tissue releases a large number of proteases that can start degrading the protein of interest. Biochemists usually include a cocktail of protease inhibitors to prevent the destruction of the protein.
Due to its potency, activity on a broad range of proteases, and low toxicity, leupeptin is frequently a component of such mixtures. Ear hairs can be destroyed by prolonged exposure to noise over 80 decibels, meningitis, ear infections, certain antibiotics and anticancer drugs, aging, and heredity (5). Studies with animal models have shown that leupeptin has promise in protecting hair cells and preventing a large degree of hearing loss after subjecting the mammals to loud noise or certain antibiotics.

