X-gal is an analog of lactose, and therefore may be hydrolyzed by the β-galactosidase enzyme which cleaves the β-glycosidic bond in D-lactose.
General Behavior
- X-GAL, when cleaved by β-galactosidase, yields galactose and 5-bromo-4-chloro-3-hydroxyindole.
- X-GAL itself is colorless, the presence of blue-colored product therefore may be used as a test for the presence of an active β-galactosidase.
- This easy identification of an active enzyme allows the gene for β-galactosidase (the lacZ gene) to be used as a reporter gene in various applications.
Gene Clone Use
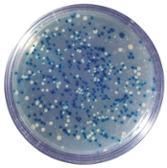
- In gene cloning, X-GAL is used as a visual indication of whether a cell expresses a functional β-galactosidase enzyme in a technique called blue/white screening.
- This method of screening is a convenient way of distinguishing a successful cloning product from other unsuccessful ones.
- The blue/white screening method relies on the principle of β±-complementation of the β-galactosidase gene, where a fragment of the lacZ gene (lacZβ±) in the plasmid can complement another mutant lacZ gene (lacZΔM15) in the cell.
- Both genes by themselves produce non-functional peptides, however, when expressed together, as when a plasmid containing lacZβ± is transformed into a lacZβ”M15 cells, they form a functional β-galactosidase.
- The presence of an active β-galactosidase may be detected when cells are grown in plates containing X-gal, the blue-colored product precipitated within cells resulted in the characteristic blue colonies.
- However, the multiple cloning site, where a gene of interest may be ligated into the plasmid vector, is located within the lacZβ± gene.
- Successful ligation therefore disrupts the lacZβ± gene, β±-complementation is therefore also disrupted and no functional β-galactosidase can form, resulting in white colonies.
- Cells containing successfully ligated insert can then be easily identified by its white coloration from the unsuccessful blue ones.
- Example of cloning vectors used for this test are pUC19, pBluescript, pGem-T Vectors, and it also requires the use of specific E. coli host strains such as DH5β± which carries the mutant lacZβ”M15 gene.
Protein-Protein Interaction
- In two-hybrid analysis, β-galactosidase may be used as a reporter to identify proteins that interact with each other.
- In this method, genome libraries may be screened for protein interaction using yeast or bacterial system.
- Where there is a successful interaction between proteins being screened, it will result to the binding of an activation domain to a promoter.
- If the promoter is linked to a lacZ gene, the production of β-galactosidase, which results in the formation of blue-pigmented colonies in the presence of X-gal, will therefore indicate a successful interaction between proteins.
- This technique may be limited to screening libraries of size of less than around 10.
- The successful cleavage of X-GAL also creates a noticeably foul odor due to the volatilization of indole.

