AG Scientific is a leading supplier of DTE reducing agent. Here we review some of the most frequently asked questions about this useful chemical.

What is DTE?
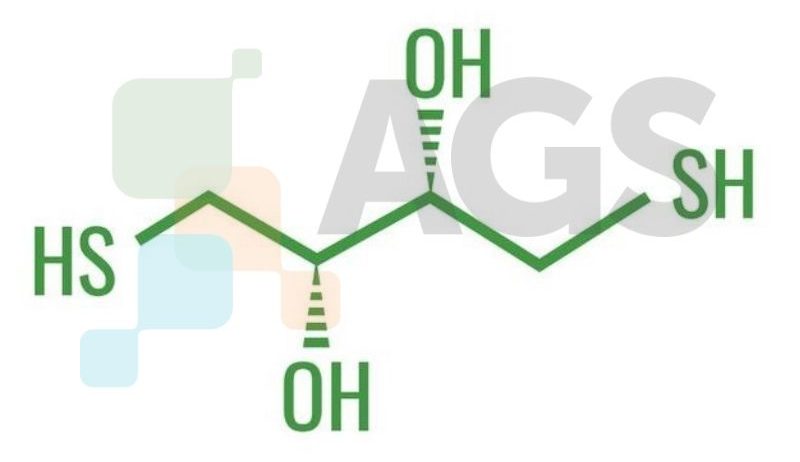
DTE (formally 1,4-Dithioerythritol) is an epimer of DTT (dithiothreitol). This sugar is derived from the corresponding 4-carbon monosaccharide erythrose and contains sulfur. It is an epimer of DTT (dithiothreitol), acting as a slightly less powerful reducing agent.Are there alternative names?
- 1,4-Dithioerythritol
- Dithioerythritol
- Cleland's Reagent
- (2R,3S)-1,4-bis(sulfanyl)butane-2,3-diol
- CAS 6892-68-8
What is the chemical formula?
C4H10O2S2 What is the molecular weight?
154.25
What does it look like?
AG Scientific's DTE is supplied as white crystals.What is the solubility of 1,4-Dithioerythritol?
This product is soluble in water and methanol, forming a clear colorless solution.What is the mechanism of action?
Reducing reagents act by giving up electrons in a chemical reaction. When these substances donate electrons, they are considered to then be oxidized. The atom/substance where the electrons are sent to is called an oxidant. The reducing agent causes the oxidant to become reduced.What are the applications of DTE?
DTE is used widely in biochemical research applications. Specifically, DTE is often used to reduce disulfide bonds to sulfhydryl groups, which it is then capable of protecting from oxidation. In this capacity, it has been used in studies researching topics from apoptosis to the use of mass spectroscopy to study nucleic acids. DTE's ability to prevent oxidation of sulfhydryl groups also makes it useful for SDS polyacrylamide gel electrophoresis. Reducing agents have important industrial applications as well, including:- Water purification
- Textile manufacturing
- Energy storage

