β-Lactamases continue to be the leading cause of resistance to β-lactam antibiotics among gram-negative bacteria. In recent years there has been an increased incidence and prevalence of extended-spectrum β-lactamases (ESBLs), enzymes that hydrolyze and cause resistance to oxyimino-cephalosporins and aztreonam.
Beta-Lactamases are the leading cause of microbial resistance to beta-lactam antibiotics in Gram-negative bacteria. AG Scientific offers a wide range of biosensor enzymes for life science research, including beta-lactamase. Our Broad Spectrum beta-Lactamase has a wide scope of activity against penicillins (penicillinase or beta I activity), up to 5th generation cephalosporins (cephalosporinase or Beta II activity), as carbapenems.
Overview
Beta-lactamases are enzymes synthesized by some bacteria that provide resistance to β-lactam antibiotics such as cephamycins, carbapenems, and penicillins. Cephalosporins are comparatively unaffected by the actions of these enzymes. Beta-lactamase provides antibiotic resistance by breaking the structure of the antibiotic.These enzymes ensure sterility of bacterial infections by eliminating false negatives via the removal of antibiotics in blood samples. These antibiotics all have a typical part in their molecular structure: a four-atom ring called a β-lactam. Through chemical reaction, the lactamase enzyme breaks the β-lactam ring open, deactivating the molecule's antibacterial properties. Beta-lactam antibiotics are usually used to treat a broad-spectrum of Gram-positive bacteria, however the development of broad-spectrum beta-lactam antibiotics have increased usefulness against Gram-negative microbes as well.
Beta-Lactamase Mechanism
Beta lactam antibiotics, like cephams and penicillin derivatives, have a four-member cyclic amide ring that inhibits the peptidoglycan layer synthesis in bacteria. A woven complex of sugar and protein pieces, these first-class bactericidal drugs destroy the integrity of the peptidoglycan layer. This method of bacterial cellular death is especially effective to Gram-positive bacteria due to the peptidoglycan layer providing the outermost resistance to the encapsulated bacterial innards.Bacteria cells undergo binary fission for asexual reproduction. The bacteria cell furrows to form a cell plate comprising of the cell wall and, consequently, the peptidoglycan layer as the cell divides into two daughter cells. Relying on penicillin-binding proteins to facilitate the synthesis of the new cell wall, this process is also called transpeptidation.
Beta lactam antibiotics are similar in structure to that of the terminal amino acid residues of the small proteins comprising the peptidoglycan matrix, and thus, act as an antagonistic drug by binding to the active site. The nature of the binding between beta lactam antibiotics and the penicillin-binding protein active sites are both irreversible and inhibitory, disrupting the formation of the peptidoglycan layer. This inhibition causes the cell to shed its cell wall, forming a spheroplast that is fragile to its environment and successfully interrupts the binary fission process.
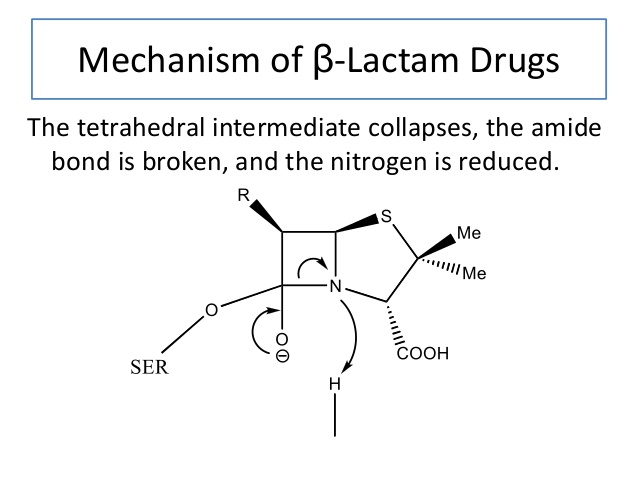
Various strains of bacteria have now developed a form of resistance to beta lactam antibiotics. Beta lactamase hydrolyzes the beta lactam ring, which characterizes beta lactam antibiotics, adding a hydroxyl group to the structure. By adding the hydroxyl group, the beta lactam ring’s structure is destroyed, and the antibiotic is rendered useless. The gene expression for beta lactamase may be induced by repeated exposure to beta-lactam antibiotics, increasing the selective pressure for bacteria to obtain the enzyme.
Induction of Beta-Lactamase Synthesis
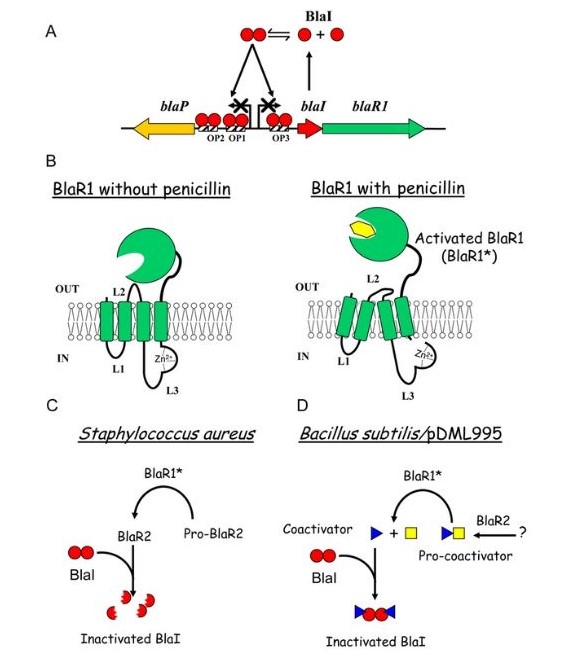
Induction of beta-lactamase synthesis is vital for the resistance of staphylococci to penicillins because the drug induces synthesis of the catalyst and is also hydrolysed by it. Some compounds, including amoxicillin and cefoxitin, both strongly induce and are hydrolysed by the chromosomally-determined beta-lactamases of Gram-negative bacilli. Additionally, other compounds, such as piperacillin and cefotaxime, are poor inducers and induction of enzyme synthesis is not necessary.
Antibiotics Inactivated by Broad-Spectrum Beta-Lactamase
| Antibiotic Class | Drugs Inactivated by B-Lactamase |
| Penicillins | Amdinocillin, Amoxicillin, Azlocillin, Benzylpenicillin, Carbenicillin, Cloxacillin,Flucloxacillin, Methicillin, Mezlocillin, Nafcillin, Oxacillin, Penicillin, Piperacillin, Ticarcillin |
| 1st Generation Cephalosporins | Cefadroxil, Cefalexin, Cefaloridine, Cefazolin |
| 2nd Generation Cephalosporins | Cefaclor, Cefamandole, Cefmetazole, Cefonicid, Cefotetan, Cefoxitin, Cefprozil, Cefuroxime |
| 3rd Generation Cephalosporins | Cefdinir, Cefixime, Cefopdoxime, Cefotaxime, Cefsulodin, Ceftazidime, Ceftibuten, Ceftiofur, Ceftizoxime, Ceftriaxone, Moxalactum |
| 4th Generation Cephalosporins | Cefepime, Cefpirome |
| 5th Generation Cephalosporins | Ceftobiprole |
| Carbapenems | Ertapenem, Imipenem, Meropenum |
Types of Beta-Lactamase Enzymes
TEM (Class A)
TEM-1 is the most common beta-lactamase in Gram-negative bacteria. They are found in E. coli and K. pneumoniae, but also found in other species of Gram-negative bacteria with increasing frequency. The extended-spectrum beta lactamase (ESBL) is the active site of the enzyme and changes its configuration to allow access to oxyimino-beta-lactam substrates. Opening the site to beta-lactam substrates additionally enhances the condition of the protein to β-lactamase inhibitors.SHV (class A)
SHV-1 shares 68 percent of its amino acids with TEM-1 and has a similar overall structure. It is found in K. pneumoniae and is responsible for up to twenty percent of the plasmid-mediated ampicillin resistance in this species.CTX-M (class A)
CTX-M represent examples of plasmid acquisition of beta-lactamase genes normally found on the chromosome of Kluyvera species, a group of rarely pathogenic commensal organisms. These enzymes are not very closely related to TEM or SHV beta-lactamases in that they show only approximately 40% identity with these two commonly isolated beta-lactamases.OXA (class D)
OXA beta-lactamases were long recognized as a less common. These beta-lactamases differ from the TEM and SHV enzymes in that they belong to molecular class D and functional group 2d.Applications
Testing Blood Culture Sterility
Blood cultures are routinely prepared in order to test for bacterial infection. False negative results might be obtained where the blood sample contains antibiotics. Incorporation of β-lactamase in the culture medium will overcome this problem when cephalosporins /penicillins are present.Environmental Monitoring of Antibiotic Manufacturing Areas
Contact plates, settle plates and air monitoring systems for testing of aseptic conditions in antibiotic manufacturing facilities need to be manufactured with agar medium for neutralization of antibiotic. This is achieved by the addition of Penicillinase or β-Lactamase to the medium. Through this, any antibiotic residues are hydrolyzed and microbial contamination can be detected.Testing for Contamination of Drugs by Antibiotics
US Code of Federal regulations states that if a reasonable possibility exists that a non-penicillin drug product has been exposed to cross-contamination with penicillin, the non-penicillin drug product shall be tested for the presence of penicillin (21 CFR 211.176, Penicillin Contamination, FDA, BY-lines No. 8 November 1977).Sterility Testing of Bulk Antibiotics
US Pharmacopeia (USP) Chapter 71 and EP Section 2.6 describe sterility testing of bulk antibiotics, which should be shown to be free from microbial contamination. The testing requires the removal of significant amounts of active antibiotic from solution by combined filtration and the use of penicillinase or β-Lactamase, The resulting solution is tested for the (lack of) growth of microbes. USP specifies that the amount of Penicillinase or β-Lactamase used in this removal process should be verified using a microbial challenge solution in a control sample.Antibiotic and Anti-Cancer Drug Development
Antibiotic discovery is one of the most groundbreaking revelations in medical history. A plethora of diseases such as bacterial meningitis, pneumonia, and septicemia, are curable via antibiotic treatment. The effectiveness of antibiotics, as well as their ability to be tolerated well by patients with low toxicity levels, makes the field of antibiotics a target for drug discovery research. Beta lactam antibiotics such as pencillin, penems, and cephalosporins contain a rigid bicyclic ring structure which functions as a means of bacterial cellular wall synthesis inhibition, causing bacterial death.In addition to the bactericidal function antibiotics possess, recent research has revealed another function: the ability to inhibit tumor cell growth. Currently, there are a multitude of anti-tumor antibiotics utilized in cancer therapy. Tumor growth inhibition occurs via DNA intercalation. Beta lactams have successfully aided chemotherapy delivery directly to tumor sites as well, emerging as potential anti-cancer drugs.
Fighting Increased Bacterial Antibiotic Resistance
Resistance to b-lactam containing antimicrobial agents continues to extend, due to the presence of beta-lactamases in Gram-negative bacterium. Over the past twenty five years, broad spectrum enzymes such as TEM and SHV variants and the Metallo β lactamases have become more prevalent.As a result of the flexibility of plasmids to still acquire extra resistance determinants, several of the beta-lactamase producing Gram-negative pathogens became multidrug resistant. In combination with decreased permeability, the organisms can become virtually untreatable with current therapies. The major teams of beta-lactamases that cause the foremost serious therapeutic issues embrace:
- the extended spectrum beta lactamases
- the plasmid mediated cephalosporinases
- The resistant TEM or SHV derived β lactamases inhibitor
- the carbapenemase producing hydrolyzed beta-lactamases
This lets beta lactamase hydrolyze the beta lactam ring in clavulanic acid, keeping the integrity of the beta lactam antibiotic’s structure and allowing beta lactam antibiotics to inhibit peptidoglycan synthesis. Carbapenems also inhibit Class A beta lactamase enzymes through hydrolysis, and are very effective against extended spectrum beta lactamases.
Additional Reading
Top 7 FAQs for Beta-LactamaseBeta-Lactamase Composition, Sterility & Stability
What Can Be Done to Fight Antibiotic Resistance?
The CRISPR/Cas9 System: A War on Antibiotic Resistance
