Boric acid, also known as hydrogen borate or boracic acid, is a weak monobasic Lewis acid of boron. Researchers may increase the acidity of boric acid through complexation reactions with polyols like sorbitol or lactitol. The compound has multiple purposes, such as applications for molecular biology in buffer preparations.
German chemist Wilhelm Homberg discovered man-made boric acid crystals in 1702 through a mixture of borax and mineral acids in water. The compound occurs in natural environments, and can be found within soil and plants. Boron, a component of this compound, plays a vital role in the growth and development of plants. This compound is generally non-toxic in natural settings and does not stay within the atmosphere, as it is usually removed by rain.
Properties of Boric Acid
Boric Acid appears as a colorless or white crystal under room temperature. The compound is highly soluble in water and has a molecular mass of 61.83 g/mol along with a pKa value between 8.92 and 9.24. It is known as sassolite in mineral form. Being a Lewis acid, the compound can accept electron pairs from a donor compound. Boric acid occurs in a planar configuration, where atoms attach themselves to the radical center, with all components positioned on the same plane.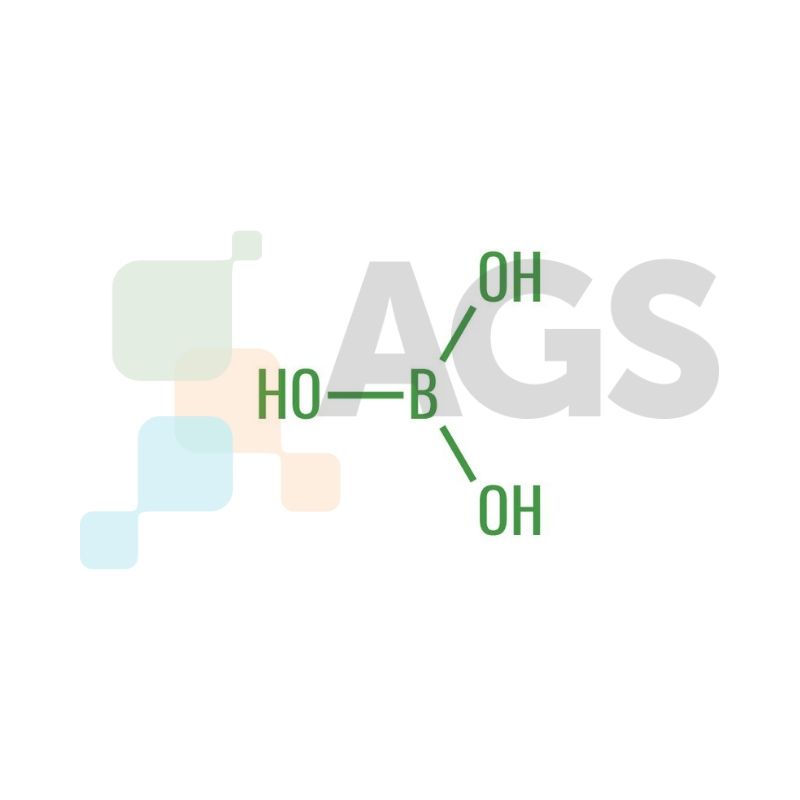
The acid has mild antimicrobial and antiseptic properties, making it a popular ingredient in manufacturing ophthalmic and dermal products. Agriculturists may apply boric acid as an effective pesticide in various forms, such as pellets and baits. Boric acid is effective as a pesticide by acting as a poison that disrupts the nervous system of insects and abrades their exoskeletal coverings. However, an excess amount of boric acid or other boron-contained compounds may lead to toxicity in plants. Common signs of boron toxicity include the yellowing of leaves and the necrosis of roots. Most crop species are highly tolerant of excess boron content, except cereal species and citrus and nut trees.
Notable Research Using Boric Acid
Topical Treatment of Pseudomonas aeruginosa
Pseudomonas aeruginosa is a Gram-negative, rod-shaped bacteria species that causes a variety of diseases in plants and animals. The bacterium functions with a pathogenic pathway involving toxic mechanisms that attack entire organisms. Researchers applied 3 percent concentrated boric acid in a topical solution against P. aeruginosa wound infections. The research resulted in significant improvements in patient recovery, without signs of adverse reactions. This compound proved an effective alternate treatment of the infection, where regular antibiotic treatment often faced resistance.Treatment of Yeast Infections
Researchers have also studied the effect of this compound on Candida albicans, an opportunistic pathogenic yeast responsible for yeast infections. The study involved exposing the yeast to boric acid concentrations, which prevented invasive hyphae development (the primary vehicle of vegetative growth). Scientists applied a broth microdilution method in measuring the boric acid sensitivity of clinical C. albicans isolates. The research involved mutant strains studied using frozen stocks with a replicator and tested with drop dilution tests. Research results concluded a slight difference in boric acid tolerance among mutant (deletion) samples, where a morphological examination attributed resistance to a specific hyperfilamentous (ultra-thin and flexible) phenotype. Specifically, exposure to boric acid led to cytoskeletal changes within C. albicans, broadening the tips of the hyphae and disrupting their directional growth. The experiment suggested this compound as a potential treatment against yeast infection, a condition that affects three out of four women.
Modification of Cotton Layers
Scientists have discovered a novel method to multifunctionally modify the structures of cotton, a fiber known for being the leading fabric worldwide. The layer-by-layer (LBL) two-stage process involved sodium lignin sulphonate, chitosan, and boric acid. LBL is an innovative textile finishing technique that results in combined functional effects. The process works through the application of two or more incompatible functional chemicals. For the experiment, scientists mixed chitosan, sodium lignin sulphonate, and boric acid due to their opposing ionic nature, adding various cotton functionalities. The experiment resulted in engineered cotton products with unique features, including UV protection, wrinkle-free texture, and antibacterial properties. Modified cotton may provide a wide range of applications, such as manufacturing hypoallergenic bedsheets for people with sensitive skin.
Multifunctional Role in Electrodisposition and Superfill
Boric acid is a crucial bath component in cobalt electroplating due to its pH buffering and surface adsorption capabilities. Research shows that this compound enables controlled depletion of protons under typical fill conditions. As such, the compound serves as a sufficient buffer that regulates pH-dependent suppression gradient and avoids rapid pinch-off. Additionally, the research proposed that boric acid adsorption delivered a two-fold effect in superfill processes. These include proton reduction, which increases deposition current efficiency, and the formation of a complex with other ions, leading to nucleation that prevents passivation.
A Catalyst in Lead-Acid Battery Function
Lead-acid batteries provide high power at an affordable rate, fueling various vehicle functions. Researchers have experimented with multiple ways of enhancing lead-acid batteries through carefully selected additives. Recent studies have suggested boric acid is a potential additive to improve lead-acid battery performance. The experiment involved full batteries and two simulated cell configurations: a three-electrode system (working electrode, a counter electrode, a Hg/Hg2SO4 reference electrode) and a Pb | electrolyte | Pb structure. Researchers added varying boric acid concentrations to the electrolytes. The experiment showed the compound's effect on the overcharging of batteries, a reaction commonly observed in lead-acid configurations. Researchers discovered that the addition of boric acid led to improved overpotential in hydrogen and oxygen evolution reaction, which reduces the effects of the battery overcharging. The study also showed that boric acid additives extended the cycling life of lead-acid batteries by introducing BO3-3 contained products with dense morphology. The reaction prevents corrosion within the configurations and may help develop the design of longer-lasting batteries.
Treatment of Chronic Wounds
Researchers studied the effects of boric acid on chronic wound treatment. The scientists administered a polyurethane sponge combined with a negative pressure wound treatment (NPWT) system for chronic wounds with defective tissues. Researchers drew comparisons with a control group of participants treated with a sponge containing silver nitrate. While both groups exhibited a decrease in wound size, participants who received the boric acid treatment showed more significant recovery, supported by increased fibroblasts, collagen synthesis, and angiogenesis (the development of new blood vessels).
Common Applications
Medical Functions
Due to boric acid's antibacterial properties, medical practitioners may use it to effectively treat skin-related conditions such as acne, athlete’s foot, or dermatologic infections. The compound’s mild antiseptic properties have made it a common component in burn dressings, mouthwashes, and diaper rash powders. Researchers and academics may include boric acid in some buffer preparations. For example, studies conducted on borate-buffered solutions indicated potent antimicrobial activity. The findings suggest borate-buffered solutions as a preferred preservative system for ophthalmic pharmaceutical agents. Specifically, traditional eye care products usually contain phosphate buffers vulnerable to infection by Gram-negative rods such as P. aeruginosa. Comparatively, exposure to boric acid preparations can significantly reduce the number of bacterial strains. By reducing bacterial proliferation, borate-buffered solutions can provide increased safety in eye care product use. This buffer compound may cause various organ-impairing symptoms within an organism, such as skin irritation and disrupted digestive systems. However, these are usually mild in human cases. Despite potential health hazards, clinical trials identify the compound as a safe and affordable medical treatment for conditions such as recurring yeast infections. Healthcare practitioners may prescribe boric acid suppositories. Studies report that the compound is well absorbed orally but results in a multifold accumulation in the bone, brain, and adipose tissue.Industrial Applications
Boric acid provides various manufacturing uses, ranging from heat-resistant glass production (due to its potentially flame-retardant properties) and LCD screens in hardening steel and in electroplating processes. The cosmetics industry adds boric acid (up to 5 percent concentration) to various products, including makeup, hair and skin treatment preparations, and shaving creams. Commercial fishing companies have used this compound as a preservative for crustacea, which reduces the effects of enzymatic darkening that alters the freshness and quality of seafood. Additionally, the application of boric acid increases the crispiness and elasticity of food products.Further Considerations
Some researchers have described boric acid as a compound that contains unusual and unique properties. A recent collaborative study highlights boric acid’s characteristics and potential use cases. These findings include a list of factors that might require consideration, such as disassociation with polymeric species (making it a weak buffer in some cases), inertness when available in a solution dosage form, and reactivity that might alter the physical and chemical stability of any molecule.Your Ally in Discovery
AG Scientific is a trusted supplier of quality scientific products for academic and research needs. Our extensive inventory includes ACS Grade Boric Acid preparations for reliable test results. Visit our site to learn how we can become your ally in discovery today.Additional Reading
- 5 Things to Consider When Choosing Biological Buffers
- Nanopore Electrochemistry: Single-Molecule Sensors
- 7 Procurement Problems in the Chemical Industry
