Dithiothreitol (DTT), also known as Cleland's reagent, is a small-molecule redox reagent. Its oxidized form is a disulfide-bonded 6-membered ring. It is used to break down protein disulfide bonds and stabilize enzymes and other proteins. DTT is a small molecule and is an epimeric compound of dithioerythritol (DTE) These reducing reagent products are readily supplied by AG Scientific, Inc.
Mechanism of Action
DTT is involved in disulfide exchange reactions. DTT is used at 1-10 mM for protein SS reduction and is capable of crossing biological membranes.Reducing properties
Dithiothreitol is a strong reducing agent with a redox potential of -0.33 V at pH 7. The reduction of a disulfide bond is followed by two sequential thiol-disulfide exchange reactions (see Fig. 2 below). The reduction generally continues past the mixed-disulfide species due to the second thiol in DTT having an increased tendency to close the ring. This causes the formation of an oxidized DTT and leaves behind a reduced disulfide bond. DTT’s reducing power is limited to pH values greater than 7, since only the negatively charged thiolate form is reactive. The thiol groups have pKa values of 9.2 and 10.1.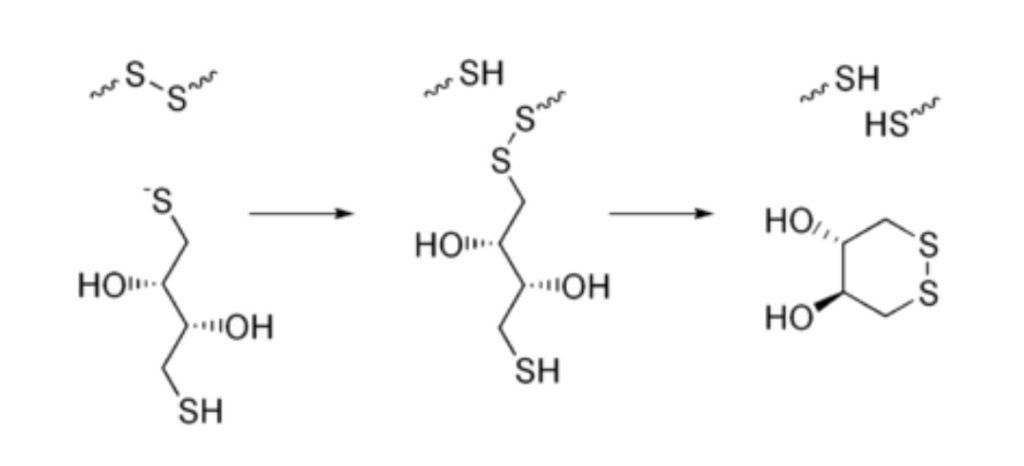
Protocols for Reducing Proteins or Peptides with DTT (Dithiothreitol)
General Protocol to Reducing Cysteines in a Protein or Peptide Solution
- Make a 1M dithiothreitol DTT stock solution in water, best to make fresh (try not to inhale the DTT).
- Add DTT to the protein or peptide solution(which is in a buffer) to a final concentration of 1mM to 10mM DTT. Incubate for 10 min. to 30 min. The reduction may work slightly better if incubating at higher temperature, such as at 37 degrees C or at 56 degrees C. However, room temperature or ice should work too.
Reducing Proteins or Peptides in Solution Prior to Alkylation
- Reduce the protein as above.
- Incubate the disulfide-reduced protein or peptide samples at an NEM molarity of at least 3-fold higher than that used for DTT. Incubate for 30 min. to 1 h.
- Dialyze away the DTT or NEM if desired.
Solubility
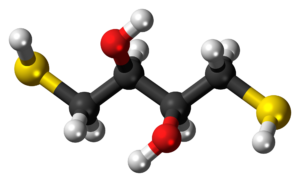
DTT is highly soluble in water, forming a clear solution. DTT is also soluble in ethanol, chloroform, ethyl acetate, and ether.
Recommended Concentrations for DTT Usage
Dithiothreitol can act as a substitute for 2-mercaptoethanol in most applications.| Concentrations to use: | Application: |
| 1-10 mM | Maintain reduced proteins in solution |
| 50-100 mM | Complete reduction for electrophoresis |
Storage & Stability
DTT solutions should be prepared fresh daily. If improperly stored (including room temperature and solution forms) its reducing ability may be reduced. Exposure to air should be minimized, even though DTT has a lower tendency to be oxidized directly by air than other reducing agents.Recorded half-life of DTT solutions at various pH and temperatures (all are in M potassium phosphate buffer):
- pH 6.5 @ 20ºC = 40 hours
- pH 7.5 @ 20ºC = 10 hours
- pH 8.5 @ 20ºC = 1.4 hours
- pH 8.5 @ 0ºC = 11 hours
- pH 8.5 @ 40ºC = 0.2 hours
- pH 8.5 @20ºC (+0.1 mM Cu2+) = 0.6 hours
- pH 8.5 @20ºC (+0.1 mM EDTA) = 4 hours
Note: DTT becomes less a less potent reducing agent with decreasing pH levels. TCEP HCl is an alternative reducing reagent which is more stable and works even at low pH. Learn more (DDT vs TCEP)
Applications of DTT
Proteomics / Protein Science
DTT is commonly used in the study of disulfide exchange reactions to reduce the disulfide bonds of proteins and reconstruct the proteins before electrophoresis analysis. The process removing DTT is performed via desalting procedures such as dialysis or gel-filtration.
DTT stops the formation of both intra- and inter-molecular disulfide bonds between cysteine residues. Disulfide bonds that are inaccessible to the solvent can be further targeted using additional denaturing techniques such as increased temperature or the addition of a powerful denaturant.
Antioxidant
As an antioxidant, DTT is used as a protective agent against ionizing radiations in living cells.Thiolated DNA
Thiolated DNA form dimers in solutions where oxygen is present causing coupling reactions to be lowered. For instance DNA can be immobilized on gold in biosensors. Dithiothreitol will react with DNA solutions and then removed using filtration or chromatography.Apoptosis
The figure below shows places where different agents influence the decomposition or inhibit the action of sodium nitroprusside and S-nitroso-N-acetylpenicillamine are indicated. Dithiothreitol accelerates the decomposition of S-nitroso-N-acetylpenicillamine and sodium nitroprusside. Hemoglobin (Hb) blocks the action of S-nitroso-N-acetylpenicillamine. Superoxide dismutase (SOD), catalase (cat) and deferoxamine (DFO) inhibit the action of sodium nitroprusside.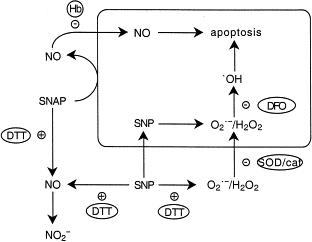
Biofuel
Studies have found DTT can be used to increase the efficiency of ethanol production from biofuels. Additional reading here: Effect of the reducing agent dithiothreitol on ethanol and acetic acid production by clostridium strain P11. Biofuels, especially bioethanol, is already used in the transportation industry as a fuel additive. Bioethanol is produced commercially from corn and other starch-rich feedstocks. In Brazil, biofuels are produced from sugar cane. There is an ongoing research on the production of bioethanol from other sugar crops.Oxidizing Agent
Dithiothreitol is used as an oxidizing agent to prevent population of mixed-disulfide species. In some instances dithiothreitol may form an adduct causing it to not cyclize since there are no free thiols remaining.Citations featuring AG Scientific's DTT
- Breeding with rare defective alleles (BRDA): a natural Populus nigra HCT mutant with modified lignin as a case study
- Development of functional amino acid-based star polymers
- The α-proteobacteria Wolbachia pipientis protein disulfide machinery has a regulatory mechanism absent in γ-proteobacteria



![Chemical structure of Dithiothreitol [DTT]](/system/user_files/wordpress/2019/05/c-1029_structure_ags.jpg)