The Wnt pathway involves a large number of proteins that can regulate the production of Wnt signaling molecules, their interactions with receptors on target cells and the physiological responses of target cells that result from the exposure of cells to the extracellular Wnt ligands. Although the presence and strength of any given effect depends on the Wnt ligand, cell type, and organism, some components of the signaling pathway are remarkably conserved in a wide variety of organisms, from Caenorhabditis elegans to humans. Protein homology suggests that several distinct Wnt ligands were present in the common ancestor of all bilaterian life, and certain aspects of Wnt signaling are present in sponges and even in slime molds.
MECHANISM
The Wnt pathway involves a large number of proteins that can regulate the production of Wnt signaling molecules, their interactions with receptors on target cells and the physiological responses of target cells that result from the exposure of cells to the extracellular Wnt ligands. Although the presence and strength of any given effect depends on the Wnt ligand, cell type, and organism, some components of the signaling pathway are remarkably conserved in a wide variety of organisms, from Caenorhabditis elegans to humans. Protein homology suggests that several distinct Wnt ligands were present in the common ancestor of all bilaterian life, and certain aspects of Wnt signaling are present in sponges and even in slime molds.
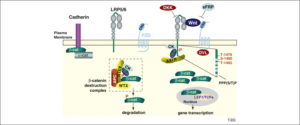
The canonical Wnt pathway describes a series of events that occur when Wnt proteins bind to cell-surface receptors of the Frizzled family, causing the receptors to activate Dishevelled family proteins and ultimately resulting in a change in the amount of beta-catenin that reaches the nucleus (Figure 2). Dishevelled (DSH) is a key component of a membrane-associated Wnt receptor complex (Figure 2), which, when activated by Wnt binding, inhibits a second complex of proteins that includes axin, GSK-3, and the protein APC(Figure 1). The axin/GSK-3/APC complex normally promotes the proteolyticdegradation of the beta-catenin intracellular signaling molecule. After this "beta-catenin destruction complex" is inhibited, a pool of cytoplasmic beta-catenin stabilizes, and some beta-catenin, is able to enter the nucleus and interact with TCF/LEF family transcription factors to promote specific gene expression.
CANCER The regulation of cell growth and survival can be subverted by a variety of genetic defects that alter transcriptional programs normally responsible for controlling cell number. High throughput analysis of these gene expression patterns should ultimately lead to the identification of minimal expression profiles that will serve as common denominators in assigning a cancer to a given category. In the course of defining the common denominators, though, we should not be too surprised to find that cancers within a single category may nevertheless exhibit seemingly disparate genetic defects. The wnt pathway has already provided an outstanding example of this. We now know of three regulatory genes in this pathway that are mutated in primary human cancers and several others that promote experimental cancers in rodents. In all of these cases the common denominator is the activation of gene transcription by beta-catenin. The resulting gene expression profile should provide us with a signature common to those cancers carrying defects in the wnt pathway. In this review, the wnt pathway will be covered from the perspective of cancer, with emphasis placed on molecular defects known to promote neoplastic transformation in humans and in animal models. There have been numerous reports on the overexpression, and sometimes underexpression, of wnt genes in human cancers, but mRNA expression levels are merely correlative. More compelling evidence, such as amplification, rearrangement, or mutation of genes encoding wnt ligands or receptors has not been forthcoming. In lieu of these sorts of findings, we are left to speculate on the consequences of epigenetic events implicating these genes in human cancer. In doing so we can use animal and cell culture models to guide our interpretation. The wnt ligands, of which there are at least 16 members in vertebrates, are secreted glycoproteins that can be loosely categorized according to their ability to promote neoplastic transformation. Sources: Lie DC, Colamarino SA, Song HJ, D©sir© L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH