IPTG is a biochemical reagent used as a molecular mimic of allolactose, a lactose metabolite that triggers transcription of the lac operon.
What is IPTG?
IPTG, known formally as Isopropyl-β-D-Thiogalactopyranoside, functions as an inducer of galactosidase activity by binding to and inhibiting the repressor. It is utilized for the induction of expression from the lac promoter and derivates.What is needed for induction?
A sterile 1 M solution of IPTG is typically added by 1:1000 dilution into a logarithmically growing bacterial culture is needed for induction. Different final concentration of IPTG may be used. Induction can be conducted using the fast method that will give you suboptimal yields or the slow method that will create more soluble proteins.Fast Induction Steps
- Pick a colony from a fresh plate and grow O/N at 30°C in a 1-2 mL LB + AMP.
- Dilute 1:50 in 2 mL LB +AMP and allow to grow for 3-4 hours at 37°C.
- Prepare 1 mL of LB +AMP and 1 mM of IPTG in a 15 mL conical. Pre-warm it 10 minutes before using it at 37°C.
- After 3-4 hrs remove 1 mL from tubes and place in labeled 1.5 mL tubes.
- Spin at max, 30 sec, RT, and remove super.
- Freeze pellet at -20°C until needed.
Slow Induction Steps
For slow induction of protein follow fast induction protocol with the following changes:- Add 20°C 1 mL LB+AMP+1 mM IPTG to 15 ml snap cap tube and incubate rotating or shaking at 20°C for 12-16 hours.
- After 12-16 hours transfer 1 mL from induced sample to labeled 1.5ml tubes
- Spin at max for 30 seconds at room temperature, and remove super
- Freeze pellet at -20°C until needed.
Where does IPTG bind?
IPTG will bind to lac repressors and liberate tetrameric repressors from the lac operator. This will allow for the transcription genes in the lac operon to catalyze the hydrolysis of ß-galactosidase into monosaccharides.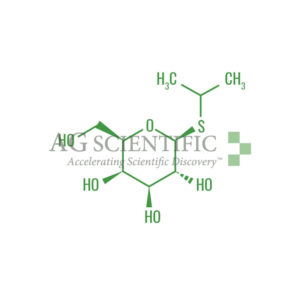
Advantages
It is used for in vivo studies due to the fact that it cannot be metabolized by E. coli. The concentrations of IPTG stay constant and as well for the rate of expression of lac p/o-controlled genes. IPTG can enter through lactose permease in low concentrations, but in high ones the cells will enter independently.
Applications
- Used in cloning procedures that require induction of β-galactosidase activity
- Used in cellular systems to study gene activity
- Used as a molecular mimic of allolactose
- Used in conjunction with X-Gal or Bluo-Gal in blue-white selection of recombinant bacterial colonies that induce expression of the lac operon in Escherichia coli
IPTG in Cloning
In cloning experiments, colonies that have been transformed with the recombinant plasmid rather than a non-recombinant need to be identified. X-gal is a substance that can be metabolised by beta-galactosidase to produce a blue product. Thus cells expressing beta-galactosidase grown in the presence of X-gal and IPTG (to induce the expression) will turn blue.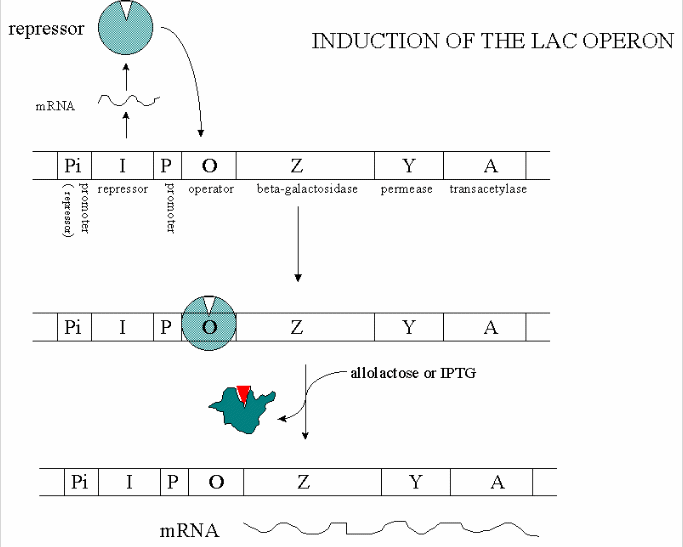
Where a DNA fragment has been inserted into the LacZ (one of the genes for beta-galactosidase) there will be no action upon X-gal and the cells will not turn blue, thus identifying the cells that carry recombinant plasmid rather than non-recombinant plasmid.

