A 12-year-old boy who was diving for a basketball in his school gym in New York City got a cut on his arm. The incident was nothing out of the ordinary, but bacteria entered Rory Staunton’s bloodstream through the cut; he developed sepsis and died three days later. The New York Times carried the story of how rapidly the infection progressed and remained undiagnosed until it was too late.
S.D. company among a few focused on innovative drugs to fight todays tougher infections
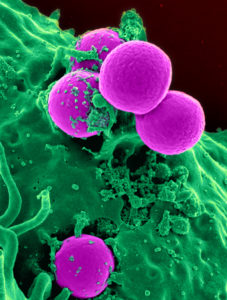
The New York Times carried the story of how rapidly the infection progressed and remained undiagnosed until it was too late.
Sepsis can be caused by pathogens, such as methicillin-resistant Staphylococcus aureus, or MRSA, a staph infection that has become immune to many common antibiotics.
Although most MRSA infections are not serious, tougher strains that resist multiple drugs can be life-threatening, requiring strong new antibiotics, of which there are few on the market.
San Diego's Trius Therapeutics is one of a handful of companies focused on developing innovative antibiotics for life-threatening diseases, including serious MRSA infections.
With bacteria, it's either we get smarter or they get smarter. The challenge for bacteria is to outsmart the antibiotic, which is why we need to come out with drugs that combat their clever ways, said Dr. Michelle Abbo, a hospitalist and internal medicine specialist with Scripps Memorial Hospital.
Last December, Trius completed the first stage-three clinical trial for the oral version of tedizolid phosphate, an antibiotic that can treat acute bacterial infections of the skin and skin structure.
It's now doing a stage-three trial for an option in which physicians can administer the drug intravenously first, then switch the patient to the oral version.
Once-a-day treatment
For reasons that are unclear, one of out of five healthy people carry the staph bacteria in their noses or in their bodies. In some people, it never shows symptoms, but it in others, it can pop up suddenly, when they get a cut or their immune system is compromised.
MRSA, a strain of staph bacteria, is carried by about 1 percent of the population. Discovered in 1961, it now resists penicillin, methicillin, amoxicillin and other antibiotics that used to kill it.
MRSA typically appears as a skin infection, spread by sharing a towel or razor, and in child-care centers and sports activities.
If acquired in a hospital, it tends to be more severe, emerging as pneumonia, or as an infection in the blood stream or a surgical site. The bacteria lives on the surface of the skin and can enter through intravenous lines and catheters.
Despite MRSAs dangers, big pharmaceutical companies have been reluctant to work on new antibiotics, fearing lackluster sales.
Sales of any single MRSA drug have topped out at $1 billion. The relatively smaller revenue scale makes it an ideal market for biotech firms to explore.
Pfizer is the only major pharma company in the field, with linezolid, approved by the U.S. Food and Drug Administration in 2000 and marketed as Zyvox, which has about $1 billion in sales annually.
Linezolid is a 600-milligram pill taken twice daily for 10 days and is the first in a new class of drugs referred to as oxazolidinones.
Trius tedizolid is a 200-milligram pill that needs to be taken only once a day for six days.
The biotech company's chief executive, Jeffrey Stein, acknowledged that linezolid has opened new horizons but said tedizolid is a better option.
Ours is fast-acting, has excellent efficacy, is a statistically improved drug. All of those are very important findings, because for skin infections, what drives prescribing behavior for doctors is safety and tolerability, Stein said.
With Zyvox, taking two large tablets twice a day for 10 days can lead to people forgetting or dropping the treatment because they start to feel better. This leads to relapse and drug resistance, which is why we have so many drug-resistant strains, such as MRSA.
Abbo, of Scripps, concurred that people do prefer shorter periods for courses of antibiotics.
It's always better to have a once-a-day drug for compliance and simplicity, she said. And tedizolid seems to cover some bugs that resist lenazolid. But there are other MRSA drugs coming down the pipeline.
The drugs she referred to, from pharma companies like Durata and Basiliea, have been in development for longer than tedizolid and therefore may have more awareness among infectious disease physicians, compared with tedizolid.
Current drugs in the market also include Boston-based Cubist Pharmaceuticals Cubicin and an Astra Zeneca collaboration for Teflaro, but it's Pfizer's Zyvox that has captured about 40 percent of the U.S. market.
Tedizolid is in the same class as linezolid, so Trius considers Pfizer's drug its main competitor.
Trius has 80 employees and does all of its research in San Diego. Its stock is listed on the Nasdaq exchange, where it's included in the biotechnology index.
Causes, diagnosis
Aside from spreading from person to person or because of hospitalization, MRSA also occurs because of overuse of antibiotics.
The risk factors for MRSA are antibiotic use, prolonged hospitalization, being in the ICU, being on dialysis, having diabetes (which makes) your immune system more compromised, Abbo said.
She has seen cases both in a hospital setting and in private practice and said that if a patient has had the infection before, medical staff take precautions, isolate the patient, pay close attention to hygiene and use gloves when touching them.
Since public health officials have been concerned about the tougher strains of MRSA for years, the number of life-threatening infections in hospitals has been coming down.
In the past few years, incidence within hospitals has fallen by 28 percent, according to a study by the Centers for Disease Control and Prevention. Rates of MRSA in the blood stream have dropped by half between 1997 and 2007, although the CDC continues to consider it a top priority.
But it's the opposite in the community, where rates continue to increase.
Defense contracts
Infectious disease physicians who used to prescribe vancomycin, an intravenous drug originally from Eli Lilly, are finding it's not as effective and have been switching over to Pfizer's Zyvox and Cubist's Cubicin. That augurs well for Trius, since its clinical trial data demonstrates that tedizolid is safer and better tolerated than Zyvox/linezolid.
Trius Stein expects tedizolid will eventually capture as big a piece of the market as linezolid has, and present a solid challenge to Pfizer's drug.
Trius is also collaborating with government agencies to develop antibiotics that counteract pathogens that could be used in bioterrorism.
It has a $28 million contract to develop novel broad-spectrum antibiotics for the National Institute of Allergy and Infectious Diseases.
It's also collaborating with Lawrence Livermore National Laboratory, tapping the lab's supercomputers for their modeling capability to synthesize compounds and test them against the lab's collection of deadly pathogens.
We can make these compounds available to the government to stockpile in case of terrorist attacks, said Stein, adding, (Trius) is a great example of another local company that relies not only on public investors but also government defense contracting as a source of revenue.
Padma Nagappan is a San Diego freelance writer. By PADMA NAGAPPAN SPECIAL TO THE U-T