Ruboxistaurin: LY333531, an orally active protein kinase C beta (PKC beta) inhibitor, is a macrocyclic bisindolylmaleimide compound under development by Eli Lilly with potential as a therapy for diabetic macular oedema and other diabetic angiopathies, including diabetic retinopathy, diabetic peripheral neuropathy and diabetic nephropathy.
Ruboxistaurin: LY333531, an orally active protein kinase C beta (PKC beta) inhibitor, is a macrocyclic bisindolylmaleimide compound under development by Eli Lilly with potential as a therapy for diabetic macular oedema and other diabetic angiopathies, including diabetic retinopathy, diabetic peripheral neuropathy and diabetic nephropathy. Ruboxistaurin is awaiting approvals in the US and Europe for the treatment of diabetic retinopathy.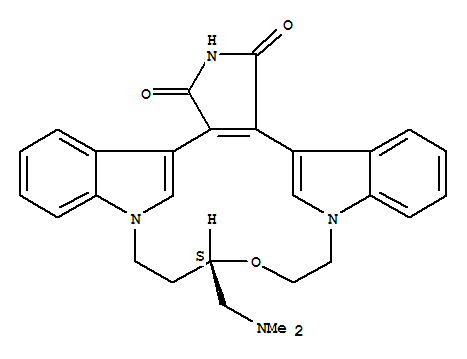 Eli Lilly and Alcon entered into a long-term agreement to co-promote ruboxistaurin in the US and Puerto Rico for diabetic retinopathy. The agreement is subject to the US FDA's approval of the agent for this indication. Under the terms of the agreement, Alcon will assume primary responsibility for promotion to eye specialists including retinal specialists and general ophthalmologists, while Eli Lilly will be targeting endocrinologists and physicians. Subject to approval in the US, Eli Lilly will receive milestone and marketing payments from Alcon. Alcon in turn will receive compensation based on product sales. In December 2003, Eli Lilly signed a joint development and co-marketing agreement with Takeda Chemical Industries for ruboxistaurin in the Japanese market. Under the terms of the agreement, Eli Lilly Japan and Takeda will jointly develop ruboxistaurin in Japan, will file an NDA for diabetic peripheral neuropathy and diabetic retinopathy, and subsequently will market the drug in Japan.
Ruboxistaurin was submitted for approval in Europe in the second quarter of 2006.
The agent is also in phase II studies for the treatment of diabetic maculopathy (macular retinopathy) in Japan.
Data from a phase III, 3-year study of ruboxistaurin in patients with moderate to severe diabetic retinopathy showed that ruboxistaurin markedly reduced the risk of sustained vision loss compared with placebo. This multicentre, randomised study, named PKC-DRS2 (Protein Kinase C-Diabetic Retinopathy Study 2), was conducted at 70 clinical sites and involved 685 patients with diabetic retinopathy.
The agent is also in a phase II study in the US, Canada and Europe in patients with clinically significant macular oedema. The trial (B7A-MC-MBCU), which will evaluate oral administration of the drug using optical coherence tomography over a period of 18 months, is expected to enrol approximately 220 patients. This randomised, double-blind, placebo-controlled study was initiated in August 2005 and is expected to be completed in March 2008.
Previously, results of the PKC-Diabetic Retinopathy Study (PKC-DRS) showed that ruboxistaurin at a dose of 32 mg/day has potential to reduce the risk of moderate vision loss especially in patients with diabetic macular oedema. This phase III, randomised, double-blind, multidose study in 252 patients with type 1 and type 2 diabetes receiving ruboxistaurin or placebo for 3-4 years evaluated the safety of the agent and its effect on progression of diabetic retinopathy, moderate vision loss and sustained moderate vision loss. The study was conducted at Joslin Diabetes Center and at centres in the US, Canada, Denmark, The Netherlands and the UK.
In 2004, Eli Lilly presented new analysis of previously reported data for ruboxistaurin in diabetic macular oedema indicating that ruboxistaurin has the potential to decrease the progression of diabetic macular oedema involving the center of the macula.
Positive results from the PKC Beta Inhibitor Diabetic Macular Edema (PKC-DMES) trial were reported in 2003.
Eli Lilly expected to file for approval of ruboxistaurin for the treatment of diabetic peripheral neuropathy in the US and Europe in 2005. However, no development was reported for this indication.
On 15 March 2007, Eli Lilly withdrew its marketing authorization application for ruboxistaurin for diabetic retinopathy filed with EMEA in May 2006. Its current development status in the EU is unclear at this stage.
Source: Adis International
Eli Lilly and Alcon entered into a long-term agreement to co-promote ruboxistaurin in the US and Puerto Rico for diabetic retinopathy. The agreement is subject to the US FDA's approval of the agent for this indication. Under the terms of the agreement, Alcon will assume primary responsibility for promotion to eye specialists including retinal specialists and general ophthalmologists, while Eli Lilly will be targeting endocrinologists and physicians. Subject to approval in the US, Eli Lilly will receive milestone and marketing payments from Alcon. Alcon in turn will receive compensation based on product sales. In December 2003, Eli Lilly signed a joint development and co-marketing agreement with Takeda Chemical Industries for ruboxistaurin in the Japanese market. Under the terms of the agreement, Eli Lilly Japan and Takeda will jointly develop ruboxistaurin in Japan, will file an NDA for diabetic peripheral neuropathy and diabetic retinopathy, and subsequently will market the drug in Japan.
Ruboxistaurin was submitted for approval in Europe in the second quarter of 2006.
The agent is also in phase II studies for the treatment of diabetic maculopathy (macular retinopathy) in Japan.
Data from a phase III, 3-year study of ruboxistaurin in patients with moderate to severe diabetic retinopathy showed that ruboxistaurin markedly reduced the risk of sustained vision loss compared with placebo. This multicentre, randomised study, named PKC-DRS2 (Protein Kinase C-Diabetic Retinopathy Study 2), was conducted at 70 clinical sites and involved 685 patients with diabetic retinopathy.
The agent is also in a phase II study in the US, Canada and Europe in patients with clinically significant macular oedema. The trial (B7A-MC-MBCU), which will evaluate oral administration of the drug using optical coherence tomography over a period of 18 months, is expected to enrol approximately 220 patients. This randomised, double-blind, placebo-controlled study was initiated in August 2005 and is expected to be completed in March 2008.
Previously, results of the PKC-Diabetic Retinopathy Study (PKC-DRS) showed that ruboxistaurin at a dose of 32 mg/day has potential to reduce the risk of moderate vision loss especially in patients with diabetic macular oedema. This phase III, randomised, double-blind, multidose study in 252 patients with type 1 and type 2 diabetes receiving ruboxistaurin or placebo for 3-4 years evaluated the safety of the agent and its effect on progression of diabetic retinopathy, moderate vision loss and sustained moderate vision loss. The study was conducted at Joslin Diabetes Center and at centres in the US, Canada, Denmark, The Netherlands and the UK.
In 2004, Eli Lilly presented new analysis of previously reported data for ruboxistaurin in diabetic macular oedema indicating that ruboxistaurin has the potential to decrease the progression of diabetic macular oedema involving the center of the macula.
Positive results from the PKC Beta Inhibitor Diabetic Macular Edema (PKC-DMES) trial were reported in 2003.
Eli Lilly expected to file for approval of ruboxistaurin for the treatment of diabetic peripheral neuropathy in the US and Europe in 2005. However, no development was reported for this indication.
On 15 March 2007, Eli Lilly withdrew its marketing authorization application for ruboxistaurin for diabetic retinopathy filed with EMEA in May 2006. Its current development status in the EU is unclear at this stage.
Source: Adis International