TGF-β superfamily members signal through a receptor complex comprising a type II and type I receptor, both serine/threonine kinases. Here, we characterize a small molecule inhibitor (SB-431542) that was identified as an inhibitor of activin receptor-like kinase (ALK)5 (the TGF-β type I receptor).
Small molecule inhibitors have proven extremely useful for investigating signal transduction pathways and have the potential for development into therapeutics for inhibiting signal transduction pathways whose activities contribute to human diseases. Transforming growth factor β (TGF-β) is a member of a large family of pleiotropic cytokines that are involved in many biological processes, including growth control, differentiation, migration, cell survival, adhesion, and specification of developmental fate, in both normal and diseased states. TGF-β superfamily members signal through a receptor complex comprising a type II and type I receptor, both serine/threonine kinases. Here, we characterize a small molecule inhibitor (SB-431542) that was identified as an inhibitor of activin receptor-like kinase (ALK)5 (the TGF-β type I receptor). We demonstrate that SB-431542 inhibits ALK5 and also the activin type I receptor ALK4 and the nodal type I receptor ALK7, which are very highly related to ALK5 in their kinase domains. It has no effect on the other, more divergent ALK family members that recognize bone morphogenetic proteins (BMPs). Consistent with this, we demonstrate that SB-431542 is a selective inhibitor of endogenous activin and TGF-β signaling but has no effect on BMP signaling. To demonstrate the specificity of SB-431542, we tested its effect on several other signal transduction pathways whose activities depend on the concerted activation of multiple kinases. SB-431542 has no effect on components of the ERK, JNK, or p38 MAP kinase pathways or on components of the signaling pathways activated in response to serum.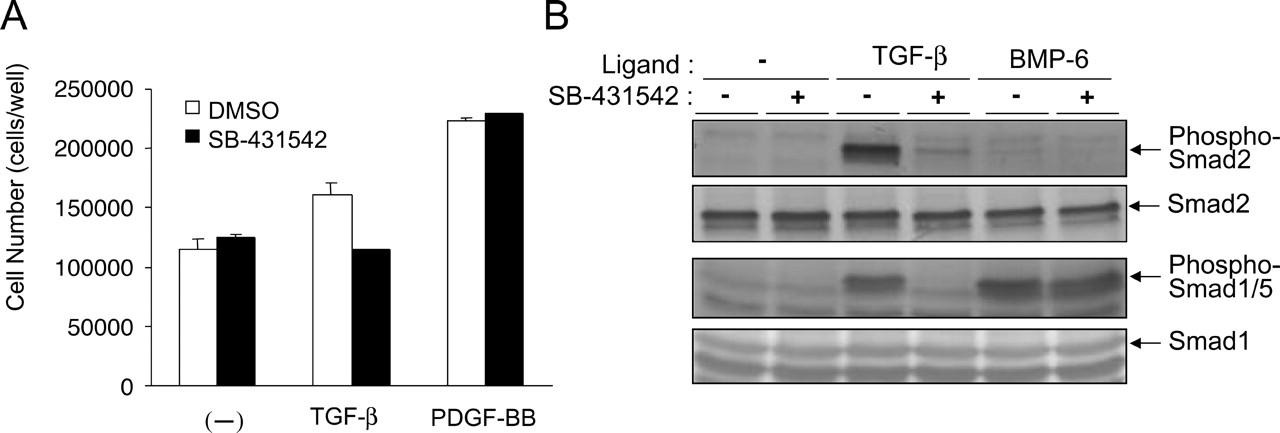
The TGF-β super family is a large family of growth and differentiation factors that regulate a wide variety of cellular processes in many different cell types and biological contexts. Different family members regulate cell proliferation (both positively and negatively), migration, extracellular matrix elaboration, adhesion, survival and differentiation, in both developing embryos and adult organisms, ranging from worms to humans (Whitman, 1998; Massagu© and Chen, 2000; Massagu© et al., 2000).
Aberrant signaling by TGF-β, the prototype of the family, has been implicated in a number of human diseases, including cancer, hereditary hemorrhagic telangiectasia, atherosclerosis, and fibrotic disease of the kidney, liver, and lung (Blobe et al., 2000). In addition, low levels of TGF-β signaling have been implicated in compromised wound healing, and inappropriately high levels of TGF-β signaling are associated with excessive scarring (Roberts and Sporn, 1993).The mechanism of signaling by TGF-β family members is now understood in some detail. The ligands bring together a type II receptor with a type I receptor, both serine/threonine kinases. The type II receptor phosphorylates and activates the type I receptor in the complex. To date, there are five mammalian type II receptors: TβR-II, ActR-II, ActR-IIB, BMPR-II, and AMHR-II and seven type I receptors (ALKs 17;Piek et al., 1999).
In most cell types, TGF-β signals through the combination of TβR-II and ALK5 (Piek et al., 1999); in endothelial cells, however, ALK1 acts as a TGF-β type I receptor (Oh et al., 2000). Activin and related ligands signal via combinations of ActR-II or ActR-IIB and ALK4, and BMPs signal through combinations of ALK2, ALK3, and ALK6 with ActR-II, ActR-IIB, or BMPR-II (Piek et al., 1999). AMH signals through a complex of AMHR-II with ALK6 (Gouedard et al., 2000), and nodal has been shown recently to signal through a complex of ActR-IIB and ALK7 (Reissmann et al., 2001).The signals are transduced to the nucleus primarily through activation of complexes of Smads. Upon activation, the type I receptors phosphorylate members of the receptor-regulated subfamily of Smads at two serines in an SSXS motif at their extreme C termini.
This activates them and enables them to form complexes with a common mediator Smad, Smad4 (Piek et al., 1999). Smads 1, 5, and 8 are substrates for ALKs 1, 2, 3, and 6, whereas Smads 2 and 3 are substrates for ALKs 4, 5, and 7 (Piek et al., 1999; Jornvall et al., 2001). The activated Smad complexes accumulate in the nucleus, where they are directly involved in the transcription of target genes, usually in association with other specific DNA-binding transcription factors (Massagu© and Wotton, 2000). In addition, TGF-β superfamily members can also induce the activation of all three known MAP kinase pathways, although the mechanism underlying this remains unclear (Massagu© and Chen, 2000).Small molecule inhibitors have been invaluable in other systems for dissecting the mechanisms of signal transduction pathways and understanding the role of individual signaling pathways in different biological processes. In addition, they have the potential to be useful for therapeutic applications (Blake et al., 2000 and references therein). Compounds that specifically inhibit receptor kinases for TGF-β superfamily members would be enormously beneficial for furthering our understanding of the mechanism of signaling and determining which biological processes require these signaling pathways.
Compounds that selectively inhibit the receptors for TGF-β, in particular, have the potential to be developed for therapeutic applications in the treatment of fibrosis, late-stage carcinogenesis, atherosclerosis, and excessive scarring (i.e., diseases in which the activity of the TGF-β signaling pathway has been implicated).A potent inhibitor of ALK5 (SB-431542) has recently been developed that acts as a competitive ATP binding site kinase inhibitor and has been shown to inhibit the in vitro phosphorylation of immobilized Smad3 with an IC50 of 94 nM (compound 14; Callahan et al., 2002). We have now investigated the efficiency of SB-431542 as an ALK5 inhibitor and rigorously tested its specificity. We demonstrate that, of the ALKs, it inhibits the activity of ALK5 and also ALK4 and ALK7, which are very similar to ALK5 in their kinase domains. It does not significantly inhibit any of the other ALKs, which have more divergent kinase domains. Consistent with this, SB-431542 inhibits TGF-β- and activin-induced phosphorylation of Smad2, which is mediated by ALK5 and ALK4, respectively, but not BMP-induced phosphorylation of Smad1, which is mediated by ALKs 2, 3, and 6. To demonstrate the specificity of SB-431542 for ALKs 4, 5, and 7, we have tested its effect on several other signaling pathways whose activities depend on the concerted activation of multiple kinases. SB-431542 had no effect on any of these signaling pathways, demonstrating that it is highly selective for these ALKs.
