Valinomycin is a potent antibiotic which acts as a potassium (K+) ionophore. Induces K+ conductivity in cell membranes. Also active in vitro against Mycobacterium Tuberculosis, and as an apoptosis inducer. Valinomycin is obtained from the cells of several Streptomyces strains, among which "S. tsusimaensis" and S. fulvissimus.
AG Scientific is a leading supplier of high-quality ionophores such as valinomycin to the in vitro diagnostic (IVD) and medical device industries.
What is Valinomycin?
It is a polymer in ringed form, which increases the permeability of K +. It is an ionophore that is capable of transporting potassium ions through the cell membrane. This ionophore is highly selective for potassium ions over sodium ions.Valinomycin comes from an antibiotic cyclododecadepsipeptide ionophore produced by Streptomyces fulvissimus and related to the enniatins. It consists of 3 moles, each with L-valine, D-alpha-hydroxy isovaleric acid, D-valine and L-lactic acid linked alternately to form a 36-member ring.
Chemical Structure
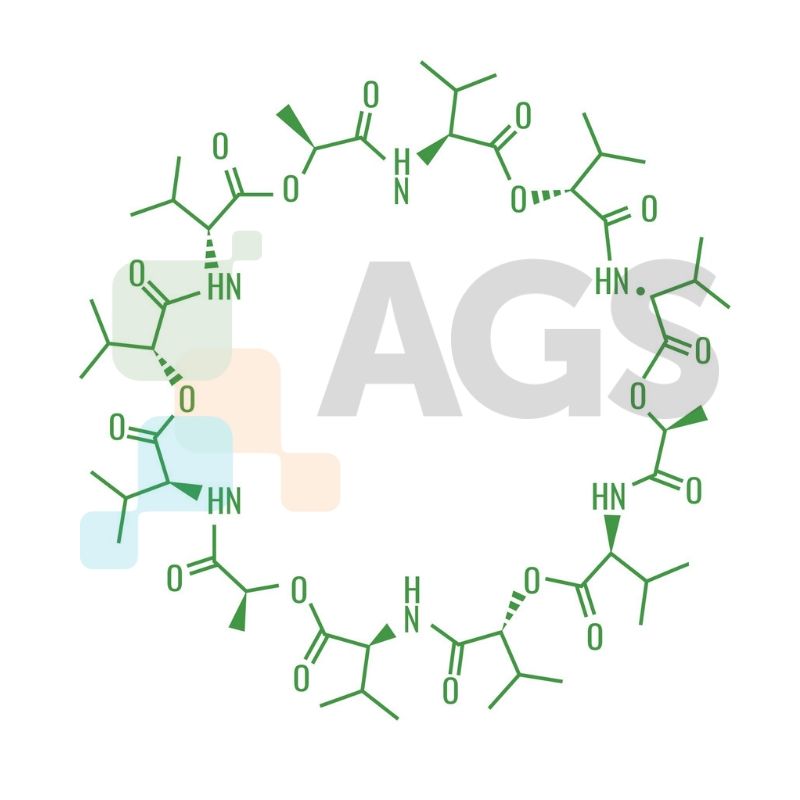
The structure of valinomycin has twelve alternating amino acids and esters that are essential for the binding of metal ions, and also for solvation in the polar solvent. The isopropyl and methyl groups are responsible for solvation in nonpolar solvents.
Applications
- Potent agent against severe acute respiratory syndrome coronavirus (SARS-CoV) in infected Vero E6 cells
- Used in experiments with membrane vesicles to create the electrochemical gradient
- Used as a nonmetallic isoforming agent in potassium selective electrodes (potassium specific transporter)
- Active in vitro against Mycobacterium tuberculosis
- Can be used to induce apoptosis
Mechanism of Action
K+ ions bind to valinomycin and echangeges across the membrane. In the absence of a K+ specific ionophore like valinomycin, K+ solely seldom crosses a lipid bilayer. In the presence of valinomycin, K+ is freely permeable.
Chemical Specifications
| Chemical Formula | C54H90N6O18 |
| CAS Number | 2001-95-8 |
| Molecular Weight | 1111.34 |
| Appearance | White crystalline powder |
| Purity | ≥93% |
| Solubility | Methanol, Ethanol |
| Product SKU | V-1013 |
Storage & Stability
When handling Valinomycin avoid dust formation, contact with skin and eyes. Properly keep containers labeled when storing at -20°C. Valinomycin is stable under normal conditions.
If skin comes in contact make sure to wash immediately with plenty of water. If it comes in contact thru the eyes, make sure to rinse under the eyelids for 15 minutes with water. If inhaled make sure to move to fresh air. Call a physician immediately. If a spill occurs make sure to use personal protective equipment to clean up.
