Pirinixic acid (Wy-14,643) is an agonistWY-14,643 - Pirinixic Acid of the peroxisome proliferator-activated receptor (PPAR) subtype α exhibiting beneficial effects in various inflammation-related processes in a slow, long-termed fashion.
- CAS No.: 50892-23-4
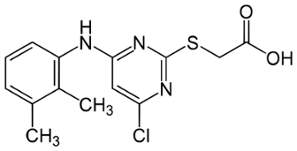
- Chemical Formula: C14H14ClN3O2S
- Synonym: Pirinixic Acid; [4-Chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid
- Appearance: White solid
- Molecular Weight: 323.8
Pirinixic acid was discovered as WY-14,643 in 1974. Pirinixic acid (Wy-14,643) is an agonist of the peroxisome proliferator-activated receptor (PPAR) subtype α exhibiting beneficial effects in various inflammation-related processes in a slow, long-termed fashion. It was developed by the pharmaceutical industry to lower serum cholesterol. It is not used in clinical applications. Known as a widely used activator of peroxisome proliferator activator receptors (PPARs). PPARs belong to the family of nuclear hormone receptor transcription factors. The potency of Wy 14643 as an activator of PPAR is species dependent, with receptor activation occurring at concentrations as low as 0.1 µM in the mouse compared to 10 µM in Xenopus. Pirinixic acid derivatives that are substituted with alkyl residues in α-position of the carboxylic group and with a 6-aminoquinoline residue at the pyrimidine moiety cause inhibition of leukotriene formation, reactive oxygen species formation, and leukocyte elastase release in response to fMLP. In parallel, Ca2+ mobilisation and the phosphorylation (activation) of p38 mitogen-activated protein kinase was significantly reduced, whereas phosphorylation of the extracellular signal-regulated kinase-2 was unaffected. Pirinixic acid itself was not or only marginally active in all these assays. Conclusively, targeted structural modification of pirinixic acid leads to bioactive compounds that display immediate anti-inflammatory properties in human neutrophils with potential therapeutic value. Sources: Daniel Poecke, Christine Greiner, Carlo Pergola, Arne Henkel, Laura Popescu, Oliver Rau, Manfred Schubert-Zsilavecz, Oliver Werz
