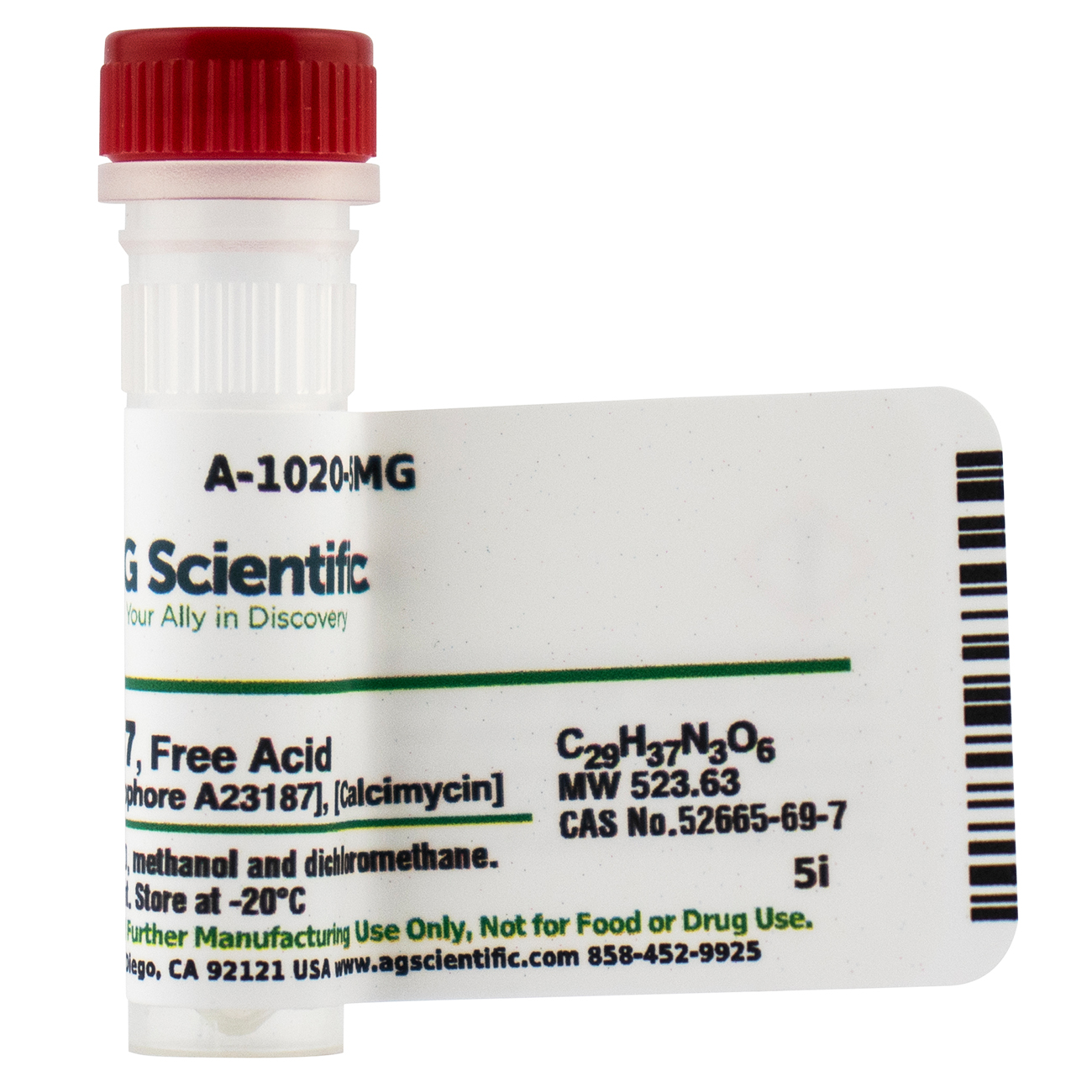
Calcium ionophore A23187; Calcimycin
52665-69-7
523.63
C29H37N3O6
DMSO, Methanol, Dicholormethane
-20°C
A23187, Free Acid is a carboxylic acid antibiotic derived from cultures of Streptomyces chartreusensis that transports divalent cations across cell membranes, which are usually impermeable to them. This mobile Ion - carrier forms stable complexes with divalent cations (ions with a charge of +2).
The ionophore is used in laboratories to increase intracellular Ca2+ levels in intact cells. A23187 has antibiotic properties against Gram-positive bacteria and fungi. A23187 also induces apoptosis in some cells (e.g. mouse lymphoma cell line, or S49, and Jurkat cells). Recent clinical studies indicate that treatment of oocytes lacking a 2ndPb with A23187 at 4.5–5.0 h after ICSI (Intracytoplasmic sperm injection) is expected to be effective at preventing fertilization failure.
0.1 lbs
Research or further manufacturing use only, not for food or drug use.