Ascomycin, also called Immunomycin, FR-900520, FK520, is an ethyl analog of tacrolimus (FK506) with strong immunosuppressant properties. Ascomycin acts by binding to immunophilins, especially macrophilin-12. It appears that Ascomycin inhibits the production of Th1 (interferon- and IL-2) and Th2 (IL-4 and IL-10) cytokines. Additionally, ascomycin preferentially inhibits the activation of mast cells, an important cellular component of the atopic response. Ascomycin produces a more selective immunomodulatory effect in that it inhibits the elicitation phase of allergic contact dermatitis but does not impair the primary immune response when administered systemically.
Ascomycin, also called Immunomycin, FR-900520, FK520, is an ethyl analog of tacrolimus (FK506) with strong immunosuppressant properties. Ascomycin acts by binding to immunophilins, especially macrophilin-12. It appears that Ascomycin inhibits the production of Th1 (interferon- and IL-2) and Th2 (IL-4 and IL-10) cytokines. Additionally, ascomycin preferentially inhibits the activation of mast cells, an important cellular component of the atopic response. Ascomycin produces a more selective immunomodulatory effect in that it inhibits the elicitation phase of allergic contact dermatitis but does not impair the primary immune response when administered systemically. ascomycin, ascomycin definition, The role of ascomycin, The applications of ascomycin, The importance of ascomycin, ascomycin features
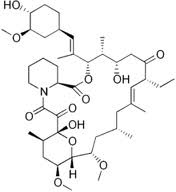 Figure X. The chemical structure of Ascomycin.
Figure X. The chemical structure of Ascomycin.
1. Promising agents for the treatment of inflammatory skin diseases
Ascomycin derivatives represent a novel class of anti-inflammatory macrolactams currently under development for the treatment of skin diseases. The main biological effect of ascomycins is an inhibition of the synthesis of both Th1 and Th2-type cytokines in target cells. Several compounds are being developed with SDZ ASM 981 being at the most advanced stage. It has high anti-inflammatory activity in animal models of skin inflammation and does not induce skin atrophy. Topical application of SDZ ASM 981 was shown to be effective in atopic dermatitis (AD), allergic contact dermatitis and also in psoriasis under semi-occlusive conditions. In patients with AD, SDZ ASM 981 cream led to consistently low systemic exposure even when applied on large areas of skin. SDZ ASM 981 overcomes the drawbacks of current topical therapies of inflammatory skin diseases as its safety profile is better than that of topical corticosteroids. Studies continue to investigate its efficacy and safety in the treatment of inflammatory skin diseases.
2. Accelerates regeneration and functional recovery.
The polyketides FK506 (tacrolimus) and FK520 (ascomycin) are potent immunosuppressants that function by inhibiting calcineurin phosphatase through formation of an FKBP12-FK506/520-calcineurin ternary complex. They also have calcineurinindependent neuroregenerative properties in cell culture and animal models of nervous system disorders. By genetic modification of the FK520 gene cluster, 13- and 15-desmethoxy analogs of FK520 that contain hydrogen, methyl, or ethyl instead of methoxy at one or both of these positions are generated. These analogs bind FKBP12 tightly, have decreased calcineurin phosphatase inhibition and immunosuppressive properties, and enhance neurite outgrowth in cell cultures. A representative compound was also shown to accelerate nerve regeneration and functional recovery in the rat sciatic nerve crush model.
3. Protects against rejection after an organ transplant.
Ascomycin is a potent immunosuppressive reagent. Immunosuppressants are also used to prevent transplant rejection in patients who receive transplanted organs. Transplant rejection occurs when the recipient's immune system does not recognize the donated organ as part of its own body. The white blood cells attack the new organ because it is identified as a harmful foreign invader. Therefore, patients who receive an organ transplant take a combination of immunosuppressants for the rest of their lives to prevent the body from destroying the new organ.
4. Antimalarial Effects of Macrolactones Related to FK520 (Ascomycin) Are Independent of the Immunosuppressive Properties of the Compounds.
FK520(Ascomycin) and a number of its nonimmunosuppressive analogues compounds were shown to be strong inhibitors of parasite growth, regardless of their immunosuppressive potency. Although some of the compounds inhibited the PPIase activity of recombinant PfFKBP35, they all inhibited the chaperone activity of this bifunctional protein. These endings suggest that the antimalarial effects of this class of drug may be mediated via inhibition of the chaperone activity rather than via the enzymatic activity of PfFKBP35. Elucidating the precise intracellular functions of PfFKBP35 may facilitate the design of more potent inhibitors that retain their specificity for parasite target protein.
SOURCES:
1. Ascomycins: promising agents for the treatment of inflammatory skin diseases. January 2000, Vol. 9, No. 1 , Pages 69-77 (doi:10.1517/13543784.9.1.69). Carle Paul, Michael Graeber, Anton Stuetz Clinical Research and Development, Novartis Pharma AG, Basel, Switzerland. carle.paul@pharma.novartis.com
2. Genetically Engineered Analogs of Ascomycin for Nerve Regeneration. W. P. Revill, J. Voda, C. R. Reeves, L. Chung, A. Schirmer, G. Ashley, J. R. Carney, M. Fardis, C. W. Carreras, Y. Zhou, L. Feng, E. Tucker, D. Robinson, and B. G. Gold. J. Pharmacol. Exp. Ther., Sep 2002; 302: 1278
3. Antimalarial Effects of Macrolactones Related to FK520 (Ascomycin) Are Independent of the Immunosuppressive Properties of the Compounds. Paul Monaghan, Maria Fardis, W. Peter Revill, and Angus Bell. The Journal of Infectious Disease, Apr 2005; 191: 1342 - 1349.