For the last 18 years, AG Scientific has been a leading supplier of DTT (dithiothreitol), historically known as Cleland's Reagent. We offer a range of catalog sizes, as well as, multi-kilogram quantities for bulk applications. Additionally, we provide a full service of bottling, sterile formulations, custom packaging, as well as, comprehensive private labeling capabilities.
DTT is a product for which we often get questions on the following: solution preparation, storage, stability, handling, trouble shooting, how to avoid common mistakes... Here are the most frequently asked questions we have received from our customers and the appropriate answers. A Material and Safety (MSDS) document link is also available for download here.
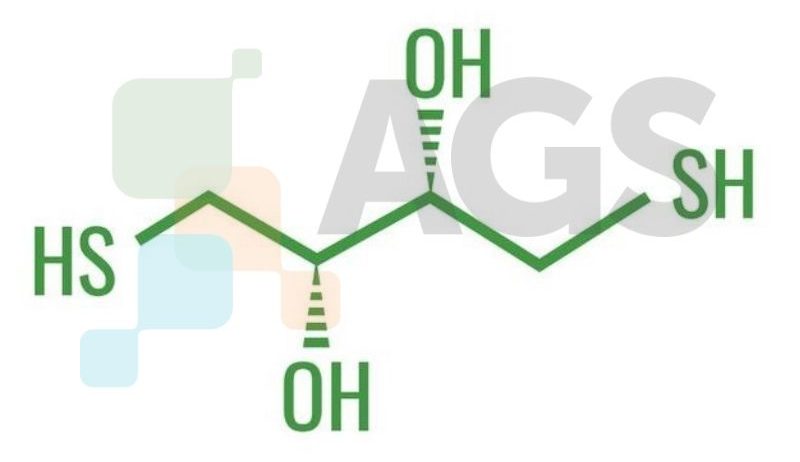
1. What is DTT?
DTT is a protective agent for reducing S-S TO SH groups. Used as a strong reducing agent for proteins and enzymes. Enhances DNA polymerase activity.
2. Are there alternative names?
- Dithiothreitol
- Cleland's reagent (discovered by W.W. Cleland, University of Wisconsin)
- CAS 3483-12-3
3. What is the chemical formula?
C4H10O2S2
4. What is the molecular weight?
286.6 Da
5. What are the recommended storage and handling conditions?
Store desiccated at 2-8ºC under inert gas.
6. Does material need to be packaged under inert gas?
Yes, argon is preferred. Nitrogen also suitable.
7. What is the stability of DTT?
When refrigerated 2-8ºC, it is stable up to 3 years. DTT is hygroscopic and has sensitivity to heat. Incompatible with strong oxidizing agents, and decomposes at pH >7.
8. How long is it stable if shipped at room temperature?
We can ensure stability for ~72 hours without significant degradation or oxidation.
9. Is there a more stable reducing agent, especially in lower pH environment?
Yes, TCEP HCl is an alternative reducing reagent with greater stability.
10. Does DTT show degradation when stressed at 30°C?
Yes, DTT demonstrates degradation starting after 3 days and increasing rapidly after 5 days at 30°C.
11. What is its melting point?
42-43ºC
12. What is its solubility?
Freely soluble in water, ethanol, acetone, ethylate, chloroform and ether. It forms a clear, colorless solution at 50 mg/mL in water. DTT has a lower volatility than that of other reducing agents such as b-mercaptoethanol. DTT is not stable in solution, thus only freshly-made DTT solutions should be used. To prepare 1 M DTT solution, dissolve 1.55 g of DTT powder in 10 mL of deionized water.
13. What is DTT's redox potential?
-0.33V at pH 7. The reducing power is limited to pH value >7.
14. What are some applications for DTT?
- Commonly used as a reducing agent or "deprotecting" agent for thiolated DNA
- Used as a reducing agent to break disulfide bonds in proteins
- At low concentrations it can be used in a reaction buffer to maintain enzymatic activities.
15. Do you offer a Molecular Biology grade DTT product?
Yes, our Dithiothreitol is Molecular Biology Grade (≥99.0% purity), free of the following: Protease, Rnase, Dnase, and ICP metals.
16. There are too many bands appearing on a Western Blot. Potential cause?
This could be caused by an aggregation of analyte. Increase the amount of DTT to ensure disulfide bonds are completely reduced (20-100 mM DTT). Heat in boiling water bath for 5-10 minutes before loading onto gel. Perform a brief centrifugation.
17. Can DTT interfere with HIS-tagged proteins?
Yes. DTT reduces nickel ions and can cause problems with purifying HIS-tagged proteins.
18. Can DTT lead to non-specific aggregation?
Yes, reducing agents like DTT are typically used to prevent the oxidation of free sulfhydryl residues (cysteines) in the protein. Such oxidation can lead to non-specific aggregation of the sample, sample heterogeneity, inactivity, or denaturation of the sample.
19. What special considerations should be made for peptides containing cysteines?
Peptides containing cysteine are prone to oxidative formation of disulfide bonds, which can form intramolecularly, forming a cyclic peptide, or intermolecularly, resulting in oligomerization. If more than one cysteine is present in a molecule, intramolecular disulfides will cause aggregation of the peptide molecules. Disulfide bonds can be reduced using DTT. However, some aggregates are very difficult to reduce. Since oxidation occurs most frequently after exposure of the deprotected peptide to air, care should be taken to keep the peptide as anaerobic as possible when multiple cysteines are present.
20. Do you have a recipe to prepare DTT stock solution?
DTT in 20 ml distilled H2O: 1 Molar 3.09 g, 0.1 Molar 0.309 g Sterilize by filtration (do not autoclave). Dispense into aliquots in microcentrifuge tubes.Store at -20°C.
21. Is this a hazardous product?
Dithiothreitol is a highly toxic substance. Harmful if inhaled or swallowed. Avoid contact with eyes, skin, and clothing. Use only in a well- ventilated area. Wash thoroughly after handling. See MSDS for full protection requirements prior to handling.
22. Who is W. Wallace Cleland?
He is often credited with discovering DTT (aka Cleland's Reagent). William Wallace Cleland was born in 1930 in Baltimore, Maryland. He received his A.B. from Oberlin College in 1950 and his M.S. and Ph.D. from the University of Wisconsin-Madison in 1953 and 1955, respectively. Cleland then did a postdoctoral fellowship at the University of Chicago, after which he returned to Madison to join the faculty of the University of Wisconsin as an Assistant Professor in 1959.
He was promoted to Associate Professor in 1962, Professor in 1966, J. Johnson Professor of Biochemistry in 1978, and Steenbock Professor of Chemical Science in 1982. Cleland remains at the University of Wisconsin where he is still actively involved in research. When Cleland first joined the faculty at the University of Wisconsin, his main focus was lipids. He spent several years studying the substrate specificity for acyl-CoA thioesters in the acylation of glycerophosphate to phosphatidic acids.
Additional Reading
- TCEP HCl vs. DTT: Preferred Reducing Agents
- Dithiothreitol (DTT) Applications You Must Know
- DTE Reducing Agent: Frequently Asked Questions
