Fidaxomicin (C52H74Cl2O18) is a member of the class of narrow spectrum macrocyclic antibiotics known as tiacumicins. The drug derives from the fermentation of tiacumicin metabolites from a soil isolate of the actinomycete Dactylosporangium aurantiacum, a Gram-positive bacterium.

Scientists discovered fidaxomicin (known as lipiarmycin) in 1975, isolating the compound in pure form from Actinoplanes deccanensis. Researchers noted the antibiotic’s effectiveness in inhibiting the growth of bacteria by interfering with RNA synthesis. Gradually, the medical field applied fidaxomicin as an effective microbial treatment, mainly against Clostridium infections.
Fidaxomicin Components and Characteristics
The antibiotic has a cyclic molecule with 18 members in its macrocyclic lactone ring and appears as a white powder under room temperature. Fidaxomicin is a unique macrolide. Unlike bacteriostatic macrolides that inhibit bacterial growth, fidaxomicin functions as a bactericidal agent. The drug metabolizes by hydrolysis and weighs 1058 g/mol.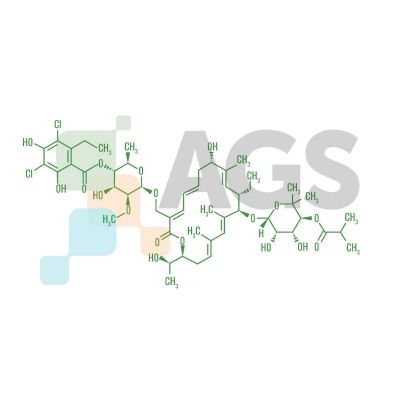
Fidaxomicin is selectively active against Gram-positive anaerobes, slightly active against Gram-positive, non-spore-forming bacilli, and poorly active against anaerobic Gram-negative non-spore bacilli. Fidaxomicin acts locally within the gastrointestinal tract with minimal absorption into the body’s circulatory systems when orally taken under medical supervision. Common fidaxomicin side effects include itching, vomiting, diarrhea, bloating, nausea, and skin rashes. Fidaxomicin may also cause serious side effects such as fever, chills, shortness of breath, and rapid heart rate.
Fidaxomicin Mechanism of Action, Uses and Research
Treats Clostridium difficile Infections
In 2011, health experts approved fidaxomicin as a treatment for Clostridium difficile, a spore-forming bacterium that causes severe diarrhea and colon inflammation (colitis). C. difficile reportedly caused diarrhea in 20–30 percent of antibiotic use cases. Fidaxomicin functions by inhibiting RNA polymerase at a unique site untargeted by other antibiotic groups, such as rifamycins. As a result, fidaxomicin reacts actively against isolates of C. difficile resistant to rifamycins and other antimicrobial classes. Specifically, the drug acts at an earlier phase in the transcription initiation pathway (where the first RNAs synthesize). The drug exhibited activity versus C. difficile with long-term antibiotic effects. Fidaxomicin’s unique target site may correlate with its limited spectrum of antimicrobial activity since sigma subunits differ among bacterial species.
Additionally, research indicates that fidaxomicin may provide better treatment outcomes than conventional antibiotics in critically ill patients suffering from C. difficile infection. The drug’s narrow spectrum of antimicrobial activity spares Gram-negative anaerobic and Gram-positive intestinal flora (posing minimal harm to positive intestinal bacteria). It thus decreases the risk of recurrence in healthcare-associated infections. While medical experts once restricted this antibiotic to adult use, the FDA approved the drug for use by children aged six months and older as oral suspension. The decision was based on a study in which 150 children affected by C. difficile received fidaxomicin. Research results showed an improved rate of sustained response in the group that received fidaxomicin treatment.
Prevents Intestinal Colonization of Vancomycin-resistant Bacteria
Conventional vancomycin treatment for C. difficile may result in colonization by pathogens within the gut because the drug disrupts intestinal flora. Researchers discovered that fidaxomicin’s macrocyclic characteristics (narrow spectrum) exhibit minimal reactions with gut microbiota, avoiding reduced resistance against opportunistic strains such as vancomycin-resistant enterococci (VRE) and extended-spectrum-β-lactamase-producing Klebsiella pneumoniae (ESBL-Kp). The mouse-model study suggested fidaxomicin as an effective alternative to vancomycin treatment, with fewer bacterial (i.e., VRE and K. pneumoniae) infection incidences.Inhibits Biofilm Development
C. difficile has a high incidence of recurrence, potentially due to the production of dormant spores in the gut of sufferers or through biofilm, both which result in persistent colonization. The biofilms of C. difficile may sporulate, leading to colonization activities. However, studies show that biofilm development requires the sporulation master regulator Spo0A. Researchers suggest that the control and management of biofilms may directly reduce sporulation activity and the risk of disease recurrence. Biofilm affects the pathogenic development of C. difficile infection, leading to bacterial proliferation and toxin production. Researchers discovered that fidaxomicin inhibits biofilm development in C. difficile, which prevents colonization in digestive systems. The experiment involved exposing sub-minimum concentrations of fidaxomicin to C. difficile. Results showed a close-dependent inhibitory effect on plankton growth and biofilm formation, suggesting fidaxomicin is a potential solution against the spread or recurrence of C. difficile infections.Potential Treatment for Zika Virus
Zika is a virus spread by Aedes mosquitoes, with symptoms such as muscle and joint pain, rashes, conjunctivitis, and fever. Severe Zika cases may cause complications such as Guillain–Barré syndrome. Scientists discovered fidaxomicin’s potential use as a therapeutic agent against Zika through its RdRp (RNA-dependent RNA polymerase)-inhibiting properties. Researchers in the experiment rounded up 1789 FDA-approved drugs and studied their effects on the Zika virus, specifically their reactions with its RdRp. The study included in vitro (through infected culture cells) and in vivo (through infected mice) observations. The experiment showed that fidaxomicin reacted effectively against Zika in in vitro and in vivo settings, particularly against the Asian and African lineage of the virus. Additionally, test results showed a wide range of health improvements in the infected rat populations, such as alleviating the usual pathological damage (for example, neuronal necrosis) that Zika sufferers experience. The findings suggest fidaxomicin is a potential antiviral therapeutic drug, specifically against Zika strains.Additional Reading
- Protease Inhibitors for COVID-19 Rapid Testing
- Avoiding Cross-Contamination in Antibiotic Manufacturing
