Sodium dodecyl sulfate is also known as SDS detergent and sodium lauryl sulfate (SLS). This detergent is an organic compound that acts as an anionic surfactant used in a variety of cleaning products. Being derived from inexpensive coconut and palm oils, it is a common component of many domestic cleaning and hygiene products.

What is Sodium Dodecyl Sulfate?
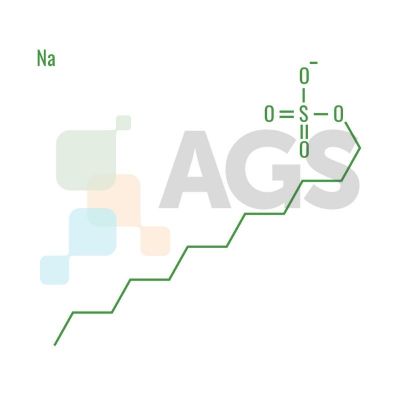
SDS is an organic compound with the chemical formula C12H25O4SNa. The salt is of an organo-sulfate consisting of a 12-carbon tail attached to a sulfate group, giving the material the amphiphilic properties required of a detergent.How is Sodium Dodecyl Suflate produced?
SDS is synthesized by treating lauryl alcohol with sulfur trioxide gas, oleum, or chlorosulfuric acid to produce hydrogen lauryl sulfate.The trioxide gas method is the typical method used at the industrial level. The resulting product is then neutralized through the addition of sodium hydroxide or sodium carbonate.
Lauryl alcohol is usually derived from either coconut or palm kernel oil by hydrolysis, which liberates their fatty acids, followed by hydrogenation. Due to this synthesis method, commercial samples of SDS are often a mixture of other alkyl sulfates, dodecyl sulfate being the main component. SDS is available commercially in powder, pellet, and solution forms. It seems the pellet form dissolves faster than the powder form in water.
SDS Detergent in the Laboratory
SDS can be used to aid in lysing cells during DNA extraction and for unraveling proteins in SDS-PAGE electrophoresis. The compound disrupts the non-covalent bonds in the proteins, causing them to denature and lose their native shape. The new negative charge is significantly greater than the original charge of the protein and the electrostatic repulsion that is created by the binding of sodium dodecyl sulfate causes the proteins to unfold into a rod-like shape. This change in conformation eliminates differences in shape as a factor for the separation of proteins in the gel.
Sodium lauryl sulfate is likely the most-researched anionic surfactant compound. Like all detergent surfactants, it removes oils from the skin and can cause skin and eye irritation. The critical micelle concentration (CMC) in pure water at 25°C is 0.0082 M and the aggregation number at this concentration is usually considered to be about 62. The micelle ionization fraction is around 0.3 (or 30%). Aqueous solutions of SDS are popular for dispersing or suspending nanotubes. Another application is for the analysis of hemoglobin. The hydrophobic group of the detergent acts upon the globin subunit, causing a conformational change. The hydrophilic group of SLS then binds with the oxidized iron subunit, producing a stable reaction product which can then be analyzed, giving a hemoglobin value which is used as part of a complete blood count.
Medical Applications of SDS Detergent
In medicine the detergent is used rectally as a laxative in enemas and as an excipient on some dissolvable aspirins and other fiber therapy caplets. Sodium Dodecyl Sulfate has also been proposed as a potential topical microbicide for intravaginal use to inhibit and potentially prevent infectino by viruses, including Herpes simplex, HIV, and Semliki Forest virus.Additional Reading
- Tips for Selecting Biological Detergents
- Detergents: Important Tools for Membrane Protein Purification
- Sulfobetaines, Zwitterionic Biodetergents in Diagnostics


