Ionophores are lipid-soluble molecules, usually synthesized by microorganisms to transport ions through a lipid bilayer of cell membrane. Ionophores facilitate ion passage in or out of cells.
Ion Transport
Ionophores function as ion carriers. Ion carriers can transfer ions from a hydrophilic medium, such as water, into a hydrophobic medium, i.e a biological membrane, where the ions typically would not be soluble. They can do this by binding to particular ions and acting as a mobile carrier, escorting them through the hydrophobic environment of cell membranes, or they can form ion channels.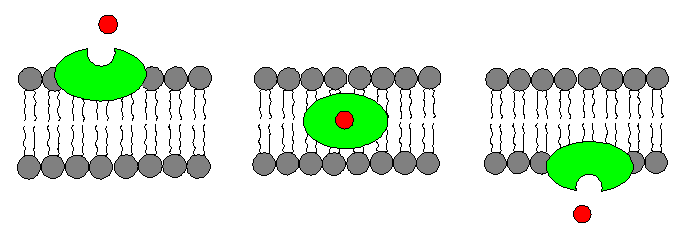
Ion channels form pores in membranes through which ions can pass. There are several types of ion channels, each regulated by various mechanisms designed to allow only certain ions to flow into and out of a cell and only at certain times.
Ion Channel Gating
Ion channels are named by their ion selectivity and by their opening/closing mechanism, which is also known as gating. Voltage-gated and ligand-gated ion channels are the most abundant and well-studied gating mechanisms, but there are also light-gated channels, mechanosensitive channels, second messenger channels, and others. Although there are some non-selective ion channels, most ion channels are selective for particular ions, allowing only ions of particular size and charge to pass through.
All endogenously present ions pass in and out of cell membranes via ion channels including calcium, potassium, sodium, chloride, and hydrogen protons. In the human body, ionophores are closely connected with functions ranging from digestion to mental health. They are used for diagnostic radio imaging, they are components of many pharmaceuticals, and are used widely in research to increase or decrease ion concentration in solution.
The ions transferred are usually metal ions, for example: lithium (Li+), sodium (Na+), potassium (K+), magnesium (Mg2+), and calcium (Ca2+); but there are ionophores that promote the transfer of other ions, such as ammonium (NH4+) amines of biological interest.
Applications
These products have have a wide variety of research applications, including in areas such as:
- Organelle behavior
- Defensive activity of bacteria
- Antibiotics
- Behaviors of synthetic bilayers
- Diagnostic radio imaging
- Pharmaceuticals
Types of Ion Transporters
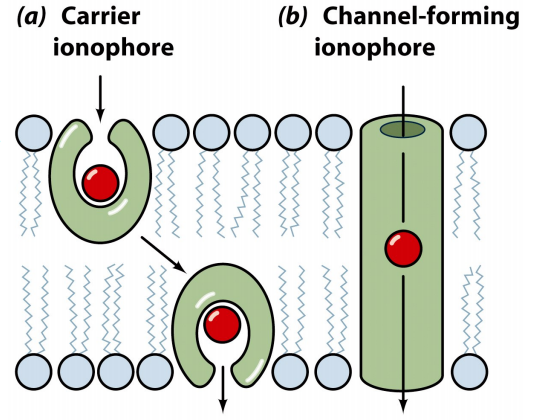
Channel Formers
Channel formers act by introducing a hydrophilic pore into the membrane, allowing the ion to pass, avoiding contact with the hydrophobic interior of the membrane. Channel forming ionophores can maintain their ion transport capacity even in low temperature conditions that may inhibit mobile ion carriers.
The average opening time of a channel is approximately one second. However, if an intense gradient is added, the channel former can transport about 20,000 cations per channel open per millisecond. This value is a thousand times greater than the number of cations that can pass in the same time interval for a single mobile ion carrier molecule.
Popular Ionophore Products
Shop our full catalog of ionophores here.
Valinomycin (V-1013)
Valinomycin is an ionophore that induces K+ conductivity in cell membranes.
• Frequently Asked Questions
Calcium Ionophore CA 1001 (E-2026)
CA 1001 has very high selectivity for Ca2+ ions. Calcium ionophore determines Ca2+ activity in membrane electrodes and is utilized for transport studies in biological membranes.
• Frequently Asked Questions
• Applications in Medical Devices
Carbonate Ionophore VII (C-8991)
Carbonate Ionophore VII is used in chromatography. It is an ion sensor compound used in ionophore potentiometric for ISE (ion-selective electrodes).
• Carbonate Ionophores for Ion Selective Electrodes
Lithium Ionophore VIII (L-1185)
Lithium Ionophore VIII is a carrier for lithium ion-selective solvent polymeric membrane electrodes. Used against Na+ and K+ cations.
• Lithium Ionophores for Ion Selective Electrodes
Nonactin (N-1155)
Nonactin is used for ammonium ion determination in potentiometric electrodes for biosensor construction
• Nonactin-based Biosensors to Measure Ammonia